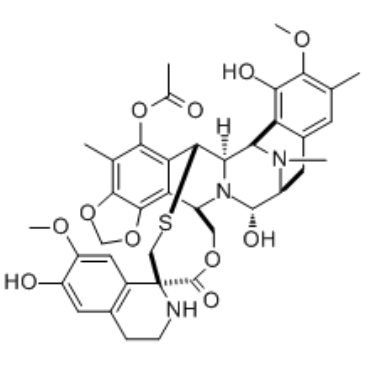
Trabectedin (also known as ET743; Ecteinascidin-743; ET-743; trade name Yondelis) is a novel antitumor agent of marine origin with potent in vitro and in vivo antitumour activity. It has been authorized as an antitumor chemotherapy drug as of 2015 for the treatment of ovarian cancer and advanced soft-tissue sarcoma. In both types of breast cancer cells, trabectedin caused cytotoxicity and apoptosis in a time- and concentration-dependent manner.
Physicochemical Properties
| Molecular Formula | C39H43N3O11S |
| Molecular Weight | 761.843 |
| Exact Mass | 761.261 |
| Elemental Analysis | C, 61.49; H, 5.69; N, 5.52; O, 23.10; S, 4.21 |
| CAS # | 114899-77-3 |
| Related CAS # | Trabectedin-d3 |
| PubChem CID | 108150 |
| Appearance | White to light yellow solid powder |
| Density | 1.6±0.1 g/cm3 |
| Index of Refraction | 1.732 |
| LogP | 3.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 54 |
| Complexity | 1450 |
| Defined Atom Stereocenter Count | 7 |
| SMILES | CC1=C(OCO2)C2=C([C@H](COC3=O)N4[C@]([C@@H]5SC[C@]63C(C=C(OC)C(O)=C7)=C7CCN6)([H])[C@@H]8N(C)[C@@H](CC9=CC(C)=C(OC)C(O)=C98)[C@@H]4O)C5=C1OC(C)=O |
| InChi Key | PKVRCIRHQMSYJX-RJZIEWFPSA-N |
| InChi Code | InChI=1S/C39H43N3O11S/c1-16-9-20-10-22-37(46)42-23-13-50-38(47)39(21-12-25(48-5)24(44)11-19(21)7-8-40-39)14-54-36(30(42)29(41(22)4)26(20)31(45)32(16)49-6)28-27(23)35-34(51-15-52-35)17(2)33(28)53-18(3)43/h9,11-12,22-23,29-30,36-37,40,44-46H,7-8,10,13-15H2,1-6H3/t22-,23-,29+,30+,36+,37-,39+/m0/s1 |
| Chemical Name | [(1R,2R,3R,11S,12S,14R,26R)-5,6',12-trihydroxy-6,7'-dimethoxy-7,21,30-trimethyl-27-oxospiro[17,19,28-trioxa-24-thia-13,30-diazaheptacyclo[12.9.6.13,11.02,13.04,9.015,23.016,20]triaconta-4(9),5,7,15,20,22-hexaene-26,1'-3,4-dihydro-2H-isoquinoline]-22-yl] acetate |
| Synonyms | Trabectedin; ET-743; ET 743; ET743; Ecteinascidin 743 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture.(3). This product is not stable in solution, please use freshly prepared working solution for optimal results. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | MX-1 cells (IC50 = 0.1 nM); MCF7 cells (IC50 = 1.5 nM); MCF7/DXR cells (IC50 = 3.7 nM); Reactive oxygen species (ROS); Apoptosis |
| ln Vitro |
Treatment with trabectedin (ET-743; 10 nM; 24-72 hours; MCF7 cells) causes cell accumulation in the late S to G2 phase[1]. Trabectedin has an IC50 of 0.1 nM, 1.5 nM, and 3.7 nM, respectively, which inhibits the growth of MX-1, MCF7, and MCF7/DXR cells[1]. Trabectedin causes cytotoxicity and apoptosis in both types of breast cancer cells in a manner that depends on both time and concentration. When Trabectedin is applied to MCF-7 cells, the expression levels of the death receptor pathway molecules TRAIL-R1/DR4, TRAIL-R2/DR5, FAS/TNFRSF6, TNF RI/TNFRSF1A, and FADD are significantly increased by 2.6, 3.1, 1.7, 11.2, and 4.0 fold, respectively. Pro-apoptotic proteins Bax, Bad, Cytochrome c, Smac/DIABLO, and cleaved Caspase-3 expression levels are increased by 4.2, 3.6, 4.8, 4.5, and 4.4 fold in MDA-MB-453 cells, whereas anti-apoptotic protein expression levels Bcl-2 and Bcl-XL are decreased by 4.8 and 5.2 fold[2]. Myxoid liposarcoma (MLS) primary tumor cultures and/or cell lines can produce CCL2, CXCL8, IL-6, VEGF, and PTX3 when treated in vitro with noncytotoxic concentrations of Trabectedin[3]. |
| ln Vivo |
Treatment with trabectin (ET-743; 30-50 μg/kg; intravenous injection; every three days; female athymic nude mice) increases the antitumor effects in nude mice bearing MX-1 mammary carcinoma xenografts without increasing toxicity[1]. Following Trabectedin treatment, CCL2, CXCL8, CD68+ infiltrating macrophages, CD31+ tumor vessels, and partial decreases in PTX3 are significantly reduced in a xenograft mouse model of human myxoid liposarcoma (MLS)[3]. |
| Cell Assay | Before samples were collected for Western blot analysis, RNAiMax Lipofectamine (4.5 mL per well in a 6-well dish) was added to siRNA targeting the EWS-FLI1 breakpoint site II, complexed, combined with cells, and incubated for 36 to 48 hours. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Administered intravenously. Biological Half-Life 33-50 hours |
| Toxicity/Toxicokinetics |
Hepatotoxicity Elevations in serum aminotransferase levels arise in almost all patients treated with trabectedin and elevations above 5 times the upper limit of normal occur 20% to 50% of patients. Pretreatment with dexamethasone appears to decrease the degree and frequency of enzyme elevations. The elevations arise within 2 to 5 days of the intravenous infusion, rise to maximal levels between 5 and 9 days and generally fall to baseline values within 3 to 4 weeks. Minor elevations in serum alkaline phosphatase and bilirubin are also common. However, clinically apparent liver injury with jaundice from trabectedin is rare. On the other hand, patients with underlying liver disease appear to be at increased risk for septicemia and multiorgan failure, and monitoring of liver tests before and during therapy is recommended. The liver injury typically mimics acute decompensation of an underlying cirrhosis with modest elevations in serum enzymes and worsening jaundice and hepatic synthetic dysfunction. Immunoallergic and autoimmune features are uncommon. Fatalities are generally due to sepsis and multiorgan failure. Likelihood score: C[HD] (probable cause of clinically apparent liver injury, generally in the setting of preexisting liver disease and use of high doses). Protein Binding 94 to 98% |
| References |
[1]. Sequence-dependent synergistic cytotoxicity of ecteinascidin-743 and NSC 125973 in human breast cancer cell linesin vitro and in vivo. Cancer Res. 2002 Dec 1;62(23):6909-15. [2]. A diverse induction of apoptosis by trabectedin in MCF-7 (HER2-/ER+) and MDA-MB-453 (HER2+/ER-) breast cancer cells. Toxicol Lett. 2013 Jun 20;221(2):128-136. [3]. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010 Mar 15;70(6):2235-44. |
| Additional Infomation |
Pharmacodynamics Two of the rings in the drug's structure allows it to covalently bind to the minor groove of DNA. The third ring protrudes from the DNA which lets it interact with nearby nuclear proteins. This has the additive effect of blocking cell division at the G2 phase. |
Solubility Data
| Solubility (In Vitro) | DMSO: 33.3~100 mg/mL (43.8~131.3 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (3.28 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (3.28 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: ≥ 2.5 mg/mL (3.28 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3126 mL | 6.5631 mL | 13.1261 mL | |
| 5 mM | 0.2625 mL | 1.3126 mL | 2.6252 mL | |
| 10 mM | 0.1313 mL | 0.6563 mL | 1.3126 mL |