Torin 2 is a novel, potent and selective ATP-competitive inhibitor of mTOR (mammalian target of rapamycin) with potential antitumor activity. It exhibits ~800-fold higher selectivity for mTOR over PI3K and has favorable pharmacokinetics. In p53−/− MEFs cell line, it inhibits mTOR with an IC50 of 0.25 nM.
Physicochemical Properties
| Molecular Formula | C24H15F3N4O |
| Molecular Weight | 432.3973 |
| Exact Mass | 432.119 |
| Elemental Analysis | C, 66.67; H, 3.50; F, 13.18; N, 12.96; O, 3.70 |
| CAS # | 1223001-51-1 |
| PubChem CID | 51358113 |
| Appearance | White to light yellow solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 623.7±55.0 °C at 760 mmHg |
| Flash Point | 331.0±31.5 °C |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.676 |
| LogP | 4.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 32 |
| Complexity | 729 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | FC(C1C([H])=C([H])C([H])=C(C=1[H])N1C(C([H])=C([H])C2=C([H])N=C3C([H])=C([H])C(C4=C([H])N=C(C([H])=C4[H])N([H])[H])=C([H])C3=C12)=O)(F)F |
| InChi Key | GUXXEUUYCAYESJ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C24H15F3N4O/c25-24(26,27)17-2-1-3-18(11-17)31-22(32)9-6-16-13-29-20-7-4-14(10-19(20)23(16)31)15-5-8-21(28)30-12-15/h1-13H,(H2,28,30) |
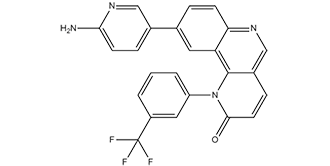
| Chemical Name | 9-(6-aminopyridin-3-yl)-1-[3-(trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2-one |
| Synonyms | Torin-2; Torin 2; Torin2 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | mTORC1; mTORC2; Autophagy; mTOR ( IC50 = 2.81 nM ); DNA-PK ( IC50 = 0.5 nM ); p110γ ( IC50 = 5.67 nM ); PI3K-C2β ( IC50 = 24.5 nM ); PI3K-C2α ( IC50 = 28.1 nM ); hVps34 ( IC50 = 8.58 nM ); PI3K ( EC50 = 200 nM ); ΡΙ4Κβ ( IC50 = 18.3 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | Using p53−/− MEFs, cellular IC50 values for mTOR are calculated. After one hour of treatment with either a vehicle or escalating concentrations of Torin 2, the cells are lysed. Using an antibody specific to phosphorylation, immunoblotting is used to monitor the phosphorylation of S6K1 Thr-389. In the meantime, phosphorylation of Akt Thr-308 in p53−/−/mLST8−/− MEFs or human PC3 cells expressing the S473D mutant of Akt1 is used to calculate cellular IC50 values for PI3Ka. | |
| Cell Assay | After treating HCT116 cells for an hour with 100 nM Torin 2 or AZD8055, they are fully cleaned using 3×PBS and 1×DMEM medium. After the specified amount of time, the cells are lysed and collected using M-PER after being incubated in DMEM medium. Protein loading is done in equal parts and protein concentrations are measured. One set of results and three repetitions of the experiment are obtained. | |
| Animal Protocol |
|
|
| References |
[1]. J Med Chem . 2011 Mar 10;54(5):1473-80. [2]. EMBO J . 2012 Mar 7;31(5):1095-108. [3]. Clin Cancer Res . 2012 Jul 1;18(13):3532-40. [4]. Cancer Cell . 2012 Jul 10;22(1):117-30. [5]. Cancer Res . 2013 Apr 15;73(8):2574-86. |
|
| Additional Infomation | Torin 2 is a member of the class of pyridoquinolines that is benzo[h][1,6]naphthyridin-2-one carrying additional 3-(trifluoromethyl)phenyl and 6-aminopyridin-3-yl substituents at positions 1 and 9 respectively. It is a potent inhibitor of mTOR and exhibits anti-cancer properties. It has a role as a mTOR inhibitor and an antineoplastic agent. It is an organofluorine compound, a pyridoquinoline, an aminopyridine and a primary amino compound. |
Solubility Data
| Solubility (In Vitro) | DMSO: 15.6~41 mg/mL (36.1~94.8 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.56 mg/mL (3.61 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 15.6 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 5%DMSO+ 40%PEG300+ 5%Tween80+ 50%ddH2O: 1.5 mg/mL (3.47mM) Solubility in Formulation 3: ≥ 2 mg/mL (4.63 mM) (saturation unknown) in 10% 1-Methyl-2-pyrrolidinone 90% PEG300 (add these co-solvents sequentially from left to right, and one by one), clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3127 mL | 11.5634 mL | 23.1267 mL | |
| 5 mM | 0.4625 mL | 2.3127 mL | 4.6253 mL | |
| 10 mM | 0.2313 mL | 1.1563 mL | 2.3127 mL |