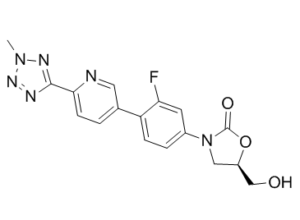
Tedizolid (formerly known as torezolid, TR-700, DA-7157, trade name Sivextro) is an oxazolidinone-class and approved antibiotic medication against Gram-positive bacteria. The mechanism of action is to inhibit protein synthesis by binding to the 50S ribosomal subunit of the G+ bacteria. Tedizolid phosphate is a phosphate ester prodrug of the active compound tedizolid. It was developed by Cubist Pharmaceuticals, following acquisition of Trius Therapeutics (formerly Dong-A Pharmaceuticals in Korea), and is approved in 2014 by the US-FDA for the treatment of acute bacterial skin and skin structure infections (also known as complicated skin and skin-structure infections (cSSSIs)).
Physicochemical Properties
| Molecular Formula | C17H15FN6O3 |
| Molecular Weight | 370.34 |
| Exact Mass | 370.118 |
| Elemental Analysis | C, 55.13; H, 4.08; F, 5.13; N, 22.69; O, 12.96 |
| CAS # | 856866-72-3 |
| Related CAS # | Tedizolid phosphate;856867-55-5;(S)-Tedizolid;1431699-67-0;Tedizolid-13C,d3; 856867-39-5 (disodium); 856866-72-3 |
| PubChem CID | 11234049 |
| Appearance | White to off-white solid powder |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 614.5±65.0 °C at 760 mmHg |
| Flash Point | 325.4±34.3 °C |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.725 |
| LogP | 1.56 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 27 |
| Complexity | 543 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | O=C1O[C@@H](CO)CN1C1C=CC(C2C=NC(C3N=NN(C)N=3)=CC=2)=C(F)C=1 |
| InChi Key | XFALPSLJIHVRKE-GFCCVEGCSA-N |
| InChi Code | InChI=1S/C17H15FN6O3/c1-23-21-16(20-22-23)15-5-2-10(7-19-15)13-4-3-11(6-14(13)18)24-8-12(9-25)27-17(24)26/h2-7,12,25H,8-9H2,1H3/t12-/m1/s1 |
| Chemical Name | (5R)-3-(3-fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenyl)-5-(hydroxymethyl)oxazolidin-2-one |
| Synonyms | DA-7157; TR700; DA7157; Tedizolid; Torezolid; 856866-72-3; TR-700; CHEBI:82717; Da-7157; UNII-97HLQ82NGL; TR-700; DA 7157; TR 700; Tedizolid; Torezolid; trade name: Sivextro |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Oxazolidinone antibacterial; MAO-A/B | ||
| ln Vitro |
Tedizolid (formerly known as torezolid, TR-700, or DA-7157, trade name Sivextro), is an oxazolidinone-class antibiotic against Gram-positive bacteria. The mechanism of action is to inhibit protein synthesis by binding to the 50S ribosomal subunit of the G+ bacteria. Tedizolid phosphate is a phosphate ester prodrug of the active compound tedizolid. It was developed by Cubist Pharmaceuticals, following acquisition of Trius Therapeutics (formerly Dong-A Pharmaceuticals in Korea), and is approved in 2014 by the US-FDA for the treatment of acute bacterial skin and skin structure infections (also known as complicated skin and skin-structure infections (cSSSIs)). Infections due to Mycobacterium abscessus carry a poor prognosis since this rapidly growing mycobacterium is intrinsically resistant to most antibiotics. Here, we evaluate the in vitro activity of the new oxazolidinone Tedizolid against a collection of 44M. abscessus clinical isolates. The MIC50s and MIC90s of Tedizolid (2 and 8μg/mL, respectively) were 2- to 16-fold lower than those of linezolid. There was no difference between the 3M. abscessus subspecies. Time-kill assays did not show any bactericidal activity at 4- and 8-fold the MIC. Combination of tedizolid with clarithromycin was synergistic against 1 out of 6 isolates, while indifferent interactions were observed for tedizolid combined with tigecycline, ciprofloxacin, and amikacin [2]. |
||
| ln Vivo |
Male ICR mice (weight, 18 to 20 g) are inoculated intraperitoneally with 1 of 4 PRSP isolates (DR9, DR10, DR11, or DR14) suspended in 10% mucin, to induce a systemic S. pneumoniae infection. The suspension contained sufficient bacteria to kill 100% of untreated control mice. At 1 h postinfection, mice receives a single dose of either tedizolid phosphate or linezolid, and survival is assessed daily for 7 days postinfection. Treatments are delivered both orally and intravenously at each of four doses (40 mg/kg of body weight, 13.33 mg/kg, 4.44 mg/kg, and 1.48 mg/kg), with 8 mice per group defined by dose, delivery method, and infecting strain. The 50% effective dose (ED50), i.e., the dose allowing survival of 50% of the infected mice, is calculated for each delivery route using probit analysis. In mice infected systemically with a lethal inoculum of PRSP 1 h prior to a single administration of either antimicrobial, oral Tedizolid phosphate was equipotent to linezolid (1 isolate) to 2-fold more potent than linezolid (3 isolates) for survival at day 7, with tedizolid phosphate 50% effective dose (ED50) values ranging from 3.19 to 11.53 mg/kg of body weight/day. In the PSSP pneumonia model, the ED50 for survival at day 15 was 2.80 mg/kg/day for oral tedizolid phosphate, whereas it was 8.09 mg/kg/day for oral linezolid following 48 h of treatment with either agent. At equivalent doses (10 mg/kg once daily tedizolid phosphate or 5 mg/kg twice daily linezolid), pneumococcal titers in the lungs at 52 h postinfection were approximately 3 orders of magnitude lower with tedizolid phosphate treatment than with linezolid treatment or no treatment. Lung histopathology showed less inflammatory cell invasion into alveolar spaces in mice treated with tedizolid phosphate than in untreated or linezolid-treated mice. These results demonstrate that tedizolid phosphate is effective in murine models of PRSP systemic infection and PSSP pneumonia [1]. |
||
| Enzyme Assay |
Susceptibility testing. [1] PRSP (penicillin G MICs ≥ 2 μg/ml) clinical isolates were collected between 2002 and 2004 from patients at a South Korean tertiary-care hospital. The species were identified by conventional methods or by using either the ID 32 GN or the ATB 32A system. MIC values of Tedizolid (active form) and linezolid against the 28 PRSP isolates were determined in agar dilution assays in accordance with NCCLS guidelines using Mueller-Hinton agar supplemented with 5% sheep blood. Serial 2-fold dilutions of stock solutions of Tedizolid or linezolid were made to yield 10× solutions that were mixed with 9 parts Mueller-Hinton agar, supplemented with 5% sheep blood, at 45 to 50°C. Final concentrations of antimicrobials were from 128 μg/ml to 0.0313 μg/ml. Agar was poured into 10-cm plastic petri dishes at a depth of 3 to 4 mm and allowed to solidify at room temperature. Inocula were prepared from single colonies of an overnight growth of PRSP isolates by suspending in broth, adjusting the turbidity to match that of the 0.5 McFarland standard, and applying 104 CFU, using a Steers replicator, onto prepared plates, starting with drug-free control plates and ending with the highest concentration of drug. Plates were incubated at 35°C for 16 to 20 h before inspection for growth. S. pneumoniae ATCC 49619 was used as a control. The MIC for each isolate was determined as the minimum concentration at which there was no growth. |
||
| Cell Assay |
MIC Determination [2] MICs of Tedizolid/TZD and LZD were determined using the broth microdilution method according to CLSI guidelines in cation-adjusted Mueller-Hinton broth (CaMHB). Briefly, TZD was solubilized in dimethylsulfoxyde (DMSO) at a concentration of 1.2 mg/L, and subsequent dilutions were performed in culture medium. Bacteria (ca. 5 × 105 CFU/mL) were inoculated into CaMHB containing 2-fold dilutions of antibiotics (0.06–64 μg/mL) in 96-well microplates. DMSO was added to LZD-containing wells at the same concentration as in TZD-containing wells (maximum concentration 5%vol/vol). MICs were determined after 3 to 5 days of incubation at 30 °C, depending on the growth of the control wells containing no antibiotic, which varied between the isolates. All MICs were performed in triplicate, and the median values are reported. Reference strain S. aureus CIP 4.83 (ATCC 6538) was used as control. Time-Kill Assays [2] Time-kill assays were performed for Tedizolid/TZD as previously described (Lefebvre et al., 2016). A bacterial suspension of M. abscessus subsp. abscessus CIP 104536T was diluted 1/1000 in fresh CaMHB and incubated for 2 h at 30 °C in a shaker incubator (180 rpm) to reach a bacterial density of 105–106 CFU/mL. TZD was then added at concentrations corresponding to 4 or 8 times its MIC, and cultures were incubated at 30 °C in an incubator shaker (180 rpm) for 72 h. CFU counts were determined at 0, 24, 48, and 72 h, using 10−1 to 10−8 dilutions plated on CaMH agar and incubated at 30 °C for 72 h. Time-kill curves were performed in duplicate. Synergy Studies [2] Synergy testing was performed using a 2-dimensional microdilution checkerboard method, as previously described (Bolhuis et al., 2014). Growth conditions were similar to those used for MIC determination, except for the Tedizolid/TZD–clarithromycin combination, for which results were read again at day 14 to monitor induction of resistance according to CLSI recommendations. The fractional inhibitory concentration index (FICI) was calculated as follows: FICI = (MICdrug A in combination/MICdrug A alone) + (MICdrug B in combination/MICdrug B alone), where drug A was TZD and drug B was clarithromycin, tigecycline, ciprofloxacin, or amikacin. Interaction between the 2 compounds was defined as synergistic when FICI value was ≤0.5, indifferent when FICI value was between 0.5 and 4, and antagonistic when FICI was >4. |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Tedizolid reaches peak plasma concentrations within three hours for oral administration and within one hour following intravenous administration; the absolute oral bioavailability is approximately 91%. Food has no effect on absorption. When given once daily, either orally or intravenously, tedizolid reaches steady-state concentrations in approximately three days. The Cmax for tedizolid after a single dose/at steady-state is 2.0 ± 0.7/2.2 ± 0.6 mcg/mL for oral administration, and 2.3 ± 0.6/3.0 ± 0.7 mcg/mL for intravenous administration, respectively. Similarly, the Tmax has a median (range) of 2.5 (1.0 - 8.0)/3.5 (1.0 - 6.0) hrs for the oral route and 1.1 (0.9 - 1.5)/1.2 (0.9 - 1.5) hrs when given intravenous. The AUC is 23.8 ± 6.8/25.6 ± 8.4 mcg\*hr/mL for oral and 26.6 ± 5.2/29.2 ± 6.2 mcg\*hr/mL for intravenous. When given as a single oral dose, approximately 82% of tedizolid is excreted via the feces and 18% in urine. The majority is found as the inactive sulphate conjugate, with only 3% recovered unchanged. Over 85% of the elimination occurs within 96 hours. The volume of distribution for tedizolid following a single intravenous dose of 200 mg is between 67 and 80 L. In a study involving oral administration of 200 mg tedizolid to steady-state, the volume of distribution was 108 ± 21 L, while a single 600 mg oral dose resulted in an apparent volume of distribution of 113.3 ± 19.3 L. Tedizolid has been observed to penetrate the interstitial space of both adipose and skeletal muscle tissue and is also found in the epithelial lining fluid as well as in alveolar macrophages. Tedizolid has an apparent oral clearance of 6.9 ± 1.7 L/hr for a single dose and 8.4 ± 2.1 L/hr at steady-state. The systemic clearance is 6.4 ± 1.2 L/hr for a single dose and 5.9 ± 1.4 L/hr at steady-state. Metabolism / Metabolites Tedizolid is administered as a phosphate prodrug that is converted to tedizolid (the circulating active moiety). Prior to excretion, the majority of tedizolid is converted to an inactive sulphate conjugate in the liver, though this is unlikely to involve the action of cytochrome P450-family enzymes. Biological Half-Life Tedizolid has a half-life of approximately 12 hours. |
||
| Toxicity/Toxicokinetics |
Hepatotoxicity Therapy with tedizolid has been associated with mild and transient elevations in serum aminotransferase and alkaline phosphatase levels in 1% to 4% of patients, although similar rates of elevations occur in patients with infections treated with comparable agents including linezolid. In all instances, the elevations occurred without symptoms or jaundice and resolved with discontinuation of the drug. While tedizolid has not been linked to cases of clinically apparent liver disease with jaundice, linezolid, a similar oxazolidinone antibiotic, has been linked to cases of lactic acidosis and systemic injury. This syndrome generally arises after 1 to 8 weeks of therapy and is sometimes associated with evidence of liver injury and jaundice. Lactic acidosis is usually due to dysfunction or loss of hepatic mitochondria, with resulting microvesicular steatosis and disturbed hepatic function (not necessarily accompanied by jaundice or even ALT or alkaline phosphatase elevations). Other serious side effects associated with mitochondrial damage include peripheral and optic neuropathy, pancreatitis, serotonin syndrome and renal injury. The mitochondrial injury is believed to be due to the inhibition of mitochondrial ribosomal function that matches the known effect of the oxazolidinones on bacterial ribosomal function. Lactic acidosis occurs after 1 to 8 weeks of treatment and can be severe, although it resolves rapidly with discontinuation. In contrast, the optic and peripheral neuropathy due to this class of antibiotics resolves more slowly and can be permanent. Lactic acidosis can be fatal, and hepatic dysfunction and jaundice have been mentioned in severe cases attributed to linezolid. This syndrome has yet to be clearly linked to tedizolid therapy. Likelihood score: E* (unproven but suspected cause of liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of tedizolid during breastfeeding. Tedizolid is 70 to 90% bound in maternal plasma, so large amounts are not expected to appear in breastmilk. If tedizolid is required by the mother, it is not a reason to discontinue breastfeeding, but because there is no published experience with tedizolid during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Approximately 70 to 90% of tedizolid is bound to human plasma proteins. |
||
| References |
[1]. Activity of Tedizolid Phosphate (TR-701) in Murine Models of Infection with Penicillin-resistant and Penicillin-sensitive Streptococcus pneumoniae.Antimicrob Agents Chemother.2012 Sep;56(9):4713-7. [2]. In vitro activity of tedizolid against the Mycobacterium abscessus complex. Diagn Microbiol Infect Dis. 2018 Mar;90(3):186-189. |
||
| Additional Infomation |
Pharmacodynamics Tedizolid is an oxazolidinone antibiotic that works by inhibiting protein synthesis by bacterial ribosomes. However, oxazolidinone antibiotics can also bind to human mitochondrial, but not cytoplasmic, ribosomes. Mitochondrial protein synthesis inhibition is associated with adverse patient effects such as neurological, hematological, and gastrointestinal toxicity, although tedizolid is tolerated better than the related [linezolid]. Alternative therapies should be considered when treating neutropenic patients with ABSSSI. _Clostridium difficile_-associated diarrhea has been reported in patients treated with tedizolid. Tedizolid phosphate is a phosphate monoester resulting from the formal condensation of equimolar amounts of phosphoric acid with the hydroxy group of tedizolid. It is a prodrug of tedizolid, used for the treatment of acute bacterial skin infections caused by certain susceptible bacteria, including Staphylococcus aureus (including methicillin-resistant strains (MRSA) and methicillin-susceptible strains), various Streptococcus species, and Enterococcus faecalis. It has a role as an antimicrobial agent, a protein synthesis inhibitor and a prodrug. It is a carbamate ester, an organofluorine compound, an oxazolidinone, a member of pyridines, a member of tetrazoles and a phosphate monoester. Drug-resistant bacteria, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and penicillin-resistant Streptococcus penumoniae, represent a massive public health threat. Tedizolid is a member of the oxazolidinone class of antibiotics, which includes the previously approved [linezolid] and is generally effective against multidrug-resistant Gram-positive bacteria. Tedizolid is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) and is generally more effective and more tolerable than [linezolid]. Tedizolid was approved by the FDA on June 20, 2014, for sale by Cubist Pharmaceuticals as tedizolid phosphate (SIVEXTRO®). This product is currently available as both an oral tablet and as a powder for intravenous injection. Tedizolid Phosphate is the phosphate salt form of tedizolid, an antibacterial agent of the oxazolidinone class, that is used for the treatment of acute bacterial skin and skin structure infections caused by certain Gram-positive microorganisms. Upon intravenous administration, tedizolid targets and binds to the 50S subunit of the bacterial ribosome. This inhibits bacterial protein synthesis. TEDIZOLID PHOSPHATE is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2014 and is indicated for skin disease caused by bacterial infection and has 7 investigational indications. Mechanism of Action Despite renewed efforts to combat the spread of antimicrobial resistance, multidrug-resistant organisms, including gram-positive bacteria such as methicillin-resistant _Staphylococcus aureus_, remain a threat. Oxazolidinones represent a relatively new class of antibacterials inhibiting protein synthesis that is generally capable of overcoming resistance to other bacterial protein synthesis inhibitors. Protein synthesis involves the action of ribosomes, multi-subunit complexes composed of both protein and ribosomal RNA (rRNA) substituents. Translocation along the length of a messenger RNA and concomitant protein synthesis involves the action of the A, P, and E sites of the peptidyltransferase centre (PTC), which accepts charged aminoacyl-tRNAs and catalyzes the formation of peptide bonds between them. The bacterial 70S ribosome comprises a small (30S) and a large (50S) subunit. Early studies into the mechanism of action of oxazolidinone antibiotics suggested that they inhibit a step in the initiation of protein synthesis. However, this mechanism was inconsistent with mapped resistance mutations, and later studies involving cross-linking and direct structural determination of the binding site revealed that oxazolidinones, including both [linezolid] and tedizolid, bind in the A site of the PTC by interacting with the 23S rRNA component. The structural studies also revealed that oxazolidinone binding alters the conformation of a conserved nucleotide in the 23S rRNA (U2585 in _Escherichia coli_), which renders the PTC non-productive for peptide bond formation. Hence, tedizolid exerts its effect through inhibiting bacterial protein synthesis. Drug Indication Tedizolid is indicated for the treatment of acute bacterial infections of the skin and skin structure (ABSSSI). To prevent drug resistance, tedizolid should only be used for infections that are caused by susceptible bacteria. Sivextro is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in adults and adolescents 12 years of age and older. In conclusion, this study showed that tedizolid was 4-fold more potent than linezolid against PRSP isolates in vitro and that tedizolid phosphate administered to mice orally or intravenously was effective in vivo against systemic infection with PRSP isolates. Oral tedizolid phosphate was also effective in vivo against pneumonia induced with a PSSP strain. These results warrant further investigation into the pharmacodynamics of tedizolid phosphate in the respiratory tract, as well as clinical evaluation of tedizolid phosphate for the treatment of pneumococcal infections. [1] This study shows that in vitro TZD activity against M. abscessus is superior to that of LZD. Intracellular TZD accumulation and synergistic activity with clarithromycin against some isolates warrant further investigations. However, the insufficient plasma concentration obtained after the recommended 200-mg dose (Cmax ≈ 2 μg/mL) (Flanagan et al., 2014) compared to MIC of the drug casts a doubt on TZD therapeutic interest for M. abscessus infections, although higher dosages might overcome this issue. Further studies are needed to explore the efficiency and toxicity of higher TZD dosages. [2] |
Solubility Data
| Solubility (In Vitro) | DMSO : ~10 mg/mL ( ~27.0 mM ) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1 mg/mL (2.70 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1 mg/mL (2.70 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 1 mg/mL (2.70 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7002 mL | 13.5011 mL | 27.0022 mL | |
| 5 mM | 0.5400 mL | 2.7002 mL | 5.4004 mL | |
| 10 mM | 0.2700 mL | 1.3501 mL | 2.7002 mL |