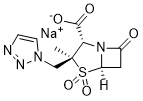
Tazobactam sodium (CL-298741; YTR-830H; CL298741; YTR830H; Zerbaxa), the sodium salt of Tazobactam, is a β-lactamase inhibitor with antibacterial activity. The combination of Tazobactam with other antibiotics such as Ceftolozane (Zerbaxa) has been used for the treatment of bacterial infections. Tazobactam is commonly used as its sodium salt, tazobactam sodium.
Physicochemical Properties
| Molecular Formula | C10H11N4NAO5S |
| Molecular Weight | 322.2708 |
| Exact Mass | 322.035 |
| Elemental Analysis | C, 37.27; H, 3.44; N, 17.39; Na, 7.13; O, 24.82; S, 9.95 |
| CAS # | 89785-84-2 |
| Related CAS # | Tazobactam;89786-04-9 |
| PubChem CID | 123630 |
| Appearance | White to off-white solid powder |
| Density | 1.92 g/cm3 |
| Boiling Point | 707.1ºC at 760 mmHg |
| Melting Point | 140-147ºC |
| Flash Point | 381.4ºC |
| LogP | -2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 20 |
| Complexity | 573 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | C(N1N=NC=C1)[C@@]1(S(=O)(=O)[C@@H]2CC(N2[C@H]1C(=O)O)=O)C.[Na] |
| InChi Key | RFMIKMMOLPNEDG-QVUDESDKSA-M |
| InChi Code | InChI=1S/C10H12N4O5S.Na/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19/h2-3,7-8H,4-5H2,1H3,(H,16,17)/q+1/p-1/t7-,8+,10+/m1./s1 |
| Chemical Name | 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-, 4,4-dioxide, sodium salt, (2S,3S,5R)- |
| Synonyms | Tazobactam sodium; CL 307579; CL-307579; CL307579; YTR 830; YTR-830; YTR830. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Tazobactam is coadministered with piperacillin or ceftolozane, pharmacokinetic information will be provided for these combinations. **Piperacillin-tazobactam** Peak plasma concentrations occur immediately after the completion of intravenous infusion. Following several doses of piperacillin-tazobactam infusions every 6 hours, peak concentrations were similar to those that were measured after the initial dose. **Ceftolozane-piperacillin** AUC: 24.4-25 mcg•h/mL Peak concentrations are reached on day 1 after the first dose and range from 18 to 18.4 mcg/mL. Tazobactam and its metabolite are mainly eliminated by the kidneys with about 80% of the administered dose eliminated as unchanged drug. The remaining drug is excreted as a single metabolite. 18.2 L when given with piperacillin 13.5-18.2 L when given with ceftolozane Piperacillin-tazobactam is widely distributed in body tissues and fluids. These may include but are not limited to the intestine, gallbladder, lung, female reproductive organs, and the bile. Meningeal distribution of piperacillin-tazobactam increases with inflammation, but is otherwise low. Because tazobactam is cleared by the kidneys and is a substrate of the transporters OAT1 and OAT3, inhibitors of these transporters should be avoided to ensure efficacy. Dosage adjustments of piperacillin-tazobactam and ceftolozane-tazobactam must be made for patients with impaired renal clearance. The mean clearance rate of tazobactam was found to be 48.3-83.6 mL/min in patients admitted to the intensive care unit who were given renal replacement therapy and receiving intravenous piperacillin-tazobactam. The clearance of tazobactam is dependent on renal function, as determined by renal clearance. Metabolism / Metabolites Tazobactam is mainly metabolized to M1, an inactive metabolite. Hydrolysis occurs on the beta-lactam ring to form M1 (the inactive metabolite). Biological Half-Life Piperacillin-tazobactam After a single dose in healthy volunteers, the plasma half-life of piperacillin and tazobactam was in the range of 0.7 to 1.2 hours. Ceftolozane-tazobactam 0.91-1.03 hours |
| Toxicity/Toxicokinetics |
Protein Binding Tazobactam is bout 30% bound to plasma proteins. |
| References |
[1]. Infect Drug Resist.2013Nov 29;6:215-23. [2]. Drugs.2014Jan;74(1):31-51 [3]. Expert Rev Anti Infect Ther. 2018;16(4):307‐320. |
| Additional Infomation |
Tazobactam is a member of the class of penicillanic acids that is sulbactam in which one of the exocyclic methyl hydrogens is replaced by a 1,2,3-triazol-1-yl group; used (in the form of its sodium salt) in combination with ceftolozane sulfate for treatment of complicated intra-abdominal infections and complicated urinary tract infections. It has a role as an antimicrobial agent, an antiinfective agent and an EC 3.5.2.6 (beta-lactamase) inhibitor. It is a member of penicillanic acids and a member of triazoles. It is functionally related to a sulbactam. It is a conjugate acid of a tazobactam(1-). Tazobactam is an antibiotic of the beta-lactamase inhibitor class that prevents the breakdown of other antibiotics by beta-lactamase enzyme producing organisms. It is combined with [Piperacillin] and [Ceftolozane] for the treatment of a variety of bacterial infections. Piperacillin-tazobactam was initially approved by the FDA in 1994, and ceftolozane-tazobactam was approved by the FDA in 2014, providing wider antibacterial coverage for gram-negative infections. In June 2019, ceftolozane-tazobactam was approved by the FDA for treating hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia, which are significant causes of morbidity and mortality in hospitalized patients. Tazobactam is a beta Lactamase Inhibitor. The mechanism of action of tazobactam is as a beta Lactamase Inhibitor. Tazobactam is a penicillanic acid sulfone derivative and beta-lactamase inhibitor with antibacterial activity. Tazobactam contains a beta-lactam ring and irreversibly binds to beta-lactamase at or near its active site. This protects other beta-lactam antibiotics from beta-lactamase catalysis. This drug is used in conjunction with beta-lactamase susceptible penicillins to treat infections caused by beta-lactamase producing organisms. A penicillanic acid and sulfone derivative and potent BETA-LACTAMASE inhibitor that enhances the activity of other anti-bacterial agents against beta-lactamase producing bacteria. Drug Indication Tazobactam is used in combination with piperacillin or ceftolozane to broaden the spectrum of piperacillin antibacterial action, treating susceptible infections. As with any other antibiotic, tazobactam should only be used for infections that are either proven or strongly suspected to be susceptible to the tazobactam containing drug. **Tazobactam-piperacillin** When combined with piperacillin, it is used to treat a variety of infections, including those caused by aerobic and facultative gram-positive and gram-negative bacteria, in addition to gram-positive and gram-negative anaerobes. Some examples of infections treated with piperacillin-tazobactam include cellulitis, diabetic foot infections, appendicitis, and postpartum endometritis infections. Certain gram-negative bacilli infections with beta-lactamase producing organisms cannot be treated with piperacillin-tazobactam, due to a gene mutation conferring antibiotic resistance. **Tazobactam-ceftolozane** Tazobactam is used in combination with [ceftolozane] for the treatment of infections caused by designated susceptible microorganisms in adult and pediatric patients: - Complicated Intra-abdominal Infections (cIAI), used in combination with [metronidazole] - Complicated Urinary Tract Infections (cUTI), including pyelonephritis - Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP) Mechanism of Action Tazobactam broadens the spectrum of piperacillin and ceftolozane by making them effective against organisms that express beta-lactamase and would normally degrade them. This occurs through the irreversible inhibition of beta-lactamase enzymes. In addition, tazobactam may bind covalently to plasmid-mediated and chromosome-mediated beta-lactamase enzymes. Tazobactam is predominantly effective against the OHIO-1, SHV-1, and TEM groups of beta-lactamases, but may also inhibit other beta-lactamases. Tazobactam shows little antibacterial activity by itself, and for this reason, is generally not administered alone. |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~65 mg/mL (~201.06 mM) Water : 65~250 mg/mL(~775.75mM ) Ethanol : ~16 mg/mL |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1030 mL | 15.5149 mL | 31.0299 mL | |
| 5 mM | 0.6206 mL | 3.1030 mL | 6.2060 mL | |
| 10 mM | 0.3103 mL | 1.5515 mL | 3.1030 mL |