TMP195 (TMP-195) is a first-in-class and selective inhibitor of class IIa histone deacetylase (HDAC) with anticancer and immunomodulatory effects. Its IC50 value for HDAC inhibition is 300 nM. By modifying macrophage phenotypes, in vivo TMP195 treatment modifies the tumor microenvironment and lowers tumor burden and pulmonary metastases. Within tumors, TMP195 promotes the recruitment and differentiation of highly stimulatory and phagocytic macrophages. Moreover, in this model, the combination of TMP195 and chemotherapy regimens or T-cell checkpoint blockade greatly improves the durability of tumor reduction. These findings present class IIa HDAC inhibition as a way to improve cancer therapy by utilizing macrophages' anti-tumor properties. In the supernatants of cultures where monocytes are differentiated into macrophages, TMP195 prevents the buildup of CCL2 protein. Comparing the vehicle group to the TMP195 group, the monocytes secrete significantly more CCL1 protein. TMP195 down- or up-regulates CCL2 and CCL1, respectively, according to the transcriptional profiling data from the PHA-stimulated PBMC experiments.
Physicochemical Properties
| Molecular Formula | C23H19F3N4O3 | |
| Molecular Weight | 456.42 | |
| Exact Mass | 456.14 | |
| Elemental Analysis | C, 60.53; H, 4.20; F, 12.49; N, 12.28; O, 10.52 | |
| CAS # | 1314891-22-9 | |
| Related CAS # |
|
|
| PubChem CID | 67324851 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Index of Refraction | 1.546 | |
| LogP | 5.57 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 9 | |
| Rotatable Bond Count | 6 | |
| Heavy Atom Count | 33 | |
| Complexity | 672 | |
| Defined Atom Stereocenter Count | 0 | |
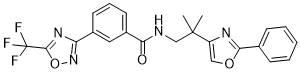
| SMILES | FC(C1=NC(C2C([H])=C([H])C([H])=C(C=2[H])C(N([H])C([H])([H])C(C([H])([H])[H])(C([H])([H])[H])C2=C([H])OC(C3C([H])=C([H])C([H])=C([H])C=3[H])=N2)=O)=NO1)(F)F |
|
| InChi Key | QTCSXAUJBQZZSN-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C23H19F3N4O3/c1-22(2,17-12-32-20(28-17)14-7-4-3-5-8-14)13-27-19(31)16-10-6-9-15(11-16)18-29-21(33-30-18)23(24,25)26/h3-12H,13H2,1-2H3,(H,27,31) | |
| Chemical Name | N-[2-methyl-2-(2-phenyl-1,3-oxazol-4-yl)propyl]-3-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]benzamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HDAC9 ( IC50 = 9 nM ); HDAC7 ( IC50 = 46 nM ); HDAC5 ( IC50 = 106 nM ); HDAC4 ( IC50 = 111 nM ); HDAC8 ( IC50 = 11700 nM ); HDAC6 ( IC50 = 47800 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | The labeled recombinant HDAC7 catalytic domain (amino acids 483-903) is applied to an arrayed library of 3,868 immobilized 20-mer peptides using DyLight 650. Using an automated TECAN HS4 microarray processing station, arrays are performed. First, blocking buffer is incubated for 30 minutes at 30°C. Next, saline containing 50 mM Tris Base and 0.1% Tween-20 (pH 7.2) is ished. Finally, the labeled HDAC7 protein is incubated for 120 minutes at 4°C. The labeled protein is pre-incubated with TMP195 for 30 minutes prior to application to the array in TMP195 competition experiments. After that, the microarrays are ished, dried, and imaged using a scanner. | |
| Cell Assay | For five days, monocytes were differentiated into antigen-presenting cells in RPMI Medium 1640 supplemented with either 0.1% (v/v) DMSO or 300 nM TMP195. Other supplements included IL-4 (10 ng/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), and GlutaMAX fetal bovine serum (10% v/v). After washing and incubating the cells in a 5 mM EDTA solution in Ca2+/Mg2+-free PBS, the cells were collected for flow cytometric analysis. | |
| Animal Protocol |
|
|
| References |
[1]. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol. 2013 May;9(5):319-25. [2]. Class IIa HDAC inhibition reduces breast tumors and metastases through anti-tumor macrophages. Nature. 2017 Mar 16;543(7645):428-432. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3 mg/mL (6.57 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 3 mg/mL (6.57 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 3 mg/mL (6.57 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly.. Solubility in Formulation 4: 5%DMSO+ Corn oil: 5.0mg/ml (10.95mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1910 mL | 10.9548 mL | 21.9096 mL | |
| 5 mM | 0.4382 mL | 2.1910 mL | 4.3819 mL | |
| 10 mM | 0.2191 mL | 1.0955 mL | 2.1910 mL |