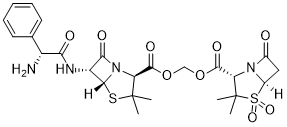
Sultamicillin (formerly known as CP-49952 and VD 1827) is an orally available, mutual prodrug form of ampicillin and sulbactam. As an antibiotic combination (co-drug or mutual prodrug) of ampicillin/sulbactam, it contains esterified ampicillin and sulbactam and is marketed under a number of trade names, e. g. Unasyn from Pfizer. Ampicillin is a semi-synthetic orally active broad spectrum antibiotic which is linked via a methylene group with a beta-lactamase inhibitor. Sultamicillin is chemically oxymethyl penicillinate sulfone ester of ampicillin. After absorption, sultamicillin releases ampicillin and sulbactam into the system, so all the antibacterial efficacy of sultamicillin is due to ampicillin and sulbactam. Ampicillin exerts antibacterial activity against sensitive organisms by inhibiting biosynthesis of cell wall mucopeptide where as sulbactam irreversibly inhibits most important beta-lactamases that occur in resistant strains.
Physicochemical Properties
| Molecular Formula | C25H30N4O9S2 | |
| Molecular Weight | 594.65 | |
| Exact Mass | 594.145 | |
| Elemental Analysis | C, 50.50; H, 5.09; N, 9.42; O, 24.21; S, 10.78 | |
| CAS # | 76497-13-7 | |
| Related CAS # | 76203-99-1 (HCl);76497-13-7 (free);83105-70-8; | |
| PubChem CID | 444022 | |
| Appearance | Solid powder | |
| Density | 1.6±0.1 g/cm3 | |
| Boiling Point | 907.7±65.0 °C at 760 mmHg | |
| Melting Point | 190° | |
| Flash Point | 502.8±34.3 °C | |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C | |
| Index of Refraction | 1.668 | |
| LogP | -0.29 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 11 | |
| Rotatable Bond Count | 9 | |
| Heavy Atom Count | 40 | |
| Complexity | 1240 | |
| Defined Atom Stereocenter Count | 6 | |
| SMILES | O=C(C(C(C)(C)S(C1C2)(=O)=O)N1C2=O)OCOC([C@@H](C(C)(C)S[C@]3([H])[C@@H]4NC([C@H](N)C5=CC=CC=C5)=O)N3C4=O)=O |
|
| InChi Key | OPYGFNJSCUDTBT-PMLPCWDUSA-N | |
| InChi Code | InChI=1S/C25H30N4O9S2/c1-24(2)17(29-20(32)16(21(29)39-24)27-19(31)15(26)12-8-6-5-7-9-12)22(33)37-11-38-23(34)18-25(3,4)40(35,36)14-10-13(30)28(14)18/h5-9,14-18,21H,10-11,26H2,1-4H3,(H,27,31)/t14-,15-,16-,17+,18+,21-/m1/s1 | |
| Chemical Name | (((2S,5R,6R)-6-((R)-2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbonyl)oxy)methyl (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam | ||
| ln Vitro | The antibacterial activity of sultamicillin against MRSA is demonstrated by a MIC value of 25 μg/mL[4]. | ||
| ln Vivo |
Sultamicillin is hydrolyzed to produce Sulbactam (HY-B0334) and Ampicillin (HY-B0522) after oral absorption[2]. When used in conjunction with an immunosuppressive medication (such as mycophenolate mofetil (HY-B0199) and methylprednisolone (HY-B0260), streptomycin (oral gavage, every 12 hours) significantly increases the survival of experimental polymicrobial sepsis in mice and improves bacterial clearance[3]. |
||
| Animal Protocol |
|
||
| References |
[1]. Sultamicillin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1989 Apr;37(4):491-522. [2]. Pharmacokinetics of sultamicillin in mice, rats, and dogs. Antimicrob Agents Chemother. 1984 May;25(5):599-602. [3]. Combined immunosuppressive and antibiotic therapy improves bacterial clearance and survival of polymicrobial septic peritonitis. Shock. 2010 Feb;33(2):155-61. [4]. Indole-based derivatives as potential antibacterial activity against methicillin-resistance Staphylococcus aureus (MRSA). Eur J Med Chem. 2020 May 15;194:112245. |
||
| Additional Infomation |
Sultamicillin is a penicillanic acid ester. It is functionally related to an ampicillin and a sulbactam. Sultamicillin has been used in trials studying the prevention and treatment of Ventilator Associated Pneumonia and Chronic Obstructive Pulmonary Disease (COPD). Sultamicillin is a combination formulation of the sodium salts of the antibiotic ampicillin and the beta-lactamase inhibitor sulbactam with antibacterial activity. Ampicillin, a broad-spectrum, semisynthetic penicillin, binds to and inactivates penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall, thereby interfering with the cross-linking of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. As a result, the cell wall is weakened and the cell lyses. The sulbactam component irreversibly binds to bacterial beta-lactamase at or near its active site, thereby interfering with substrate binding and inhibiting bacterial metabolism of penicillin and cephalosporin beta-lactam antibiotics, effectively extending their antibiotic spectrum to include many beta-lactam-resistant bacteria. |
Solubility Data
| Solubility (In Vitro) | DMSO: ~100 mg/mL (~168.16 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.20 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (4.20 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (4.20 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly.. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (4.20 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6817 mL | 8.4083 mL | 16.8166 mL | |
| 5 mM | 0.3363 mL | 1.6817 mL | 3.3633 mL | |
| 10 mM | 0.1682 mL | 0.8408 mL | 1.6817 mL |