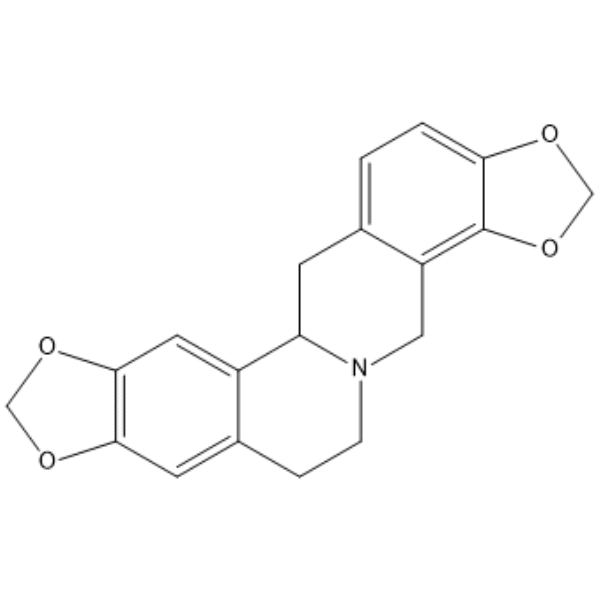
Stylopine [(±)-Stylopine; Tetrahydrocoptisine; (R,S±)-Stylopine; NSC-110382; NSC-404529] is a naturally occurring isoquinoline alkaloid isolated from C. impatiens with anti-inflammatory and antioxidant activities. Stylopine is a potent AKR1C3 (Aldo-keto reductase 103) inhibitor that significantly inhibits AKR1C3 in intact cells without a considerable cytotoxic effect. It also inhibits LPS-induced NF-κB activation and production of nitric oxide (NO), TNF-α, and IL-6 in isolated mouse peritoneal macrophages when used at concentrations ranging from 0.001 to 1 µg/ml. Tetrahydrocoptisine (10 and 30 mg/kg) inhibits xylene-induced ear edema in mice, and it decreases serum levels of TNF-α in a mouse model of LPS-induced septic shock. It reduces the severity of ethanol-induced gastric ulcers in mice when administered at doses of 10 or 20 mg/kg.
Physicochemical Properties
| Molecular Formula | C19H17NO4 |
| Molecular Weight | 323.34258 |
| Exact Mass | 323.115 |
| Elemental Analysis | C, 70.58; H, 5.30; N, 4.33; O, 19.79 |
| CAS # | 7461-02-1 |
| Related CAS # | 4312-32-7 or 7461-02-1 (racemate) ; 84-39-9 (S-isomer) |
| PubChem CID | 6770 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 466.6±34.0 °C at 760 mmHg |
| Melting Point | 221-222ºC |
| Flash Point | 142.5±22.9 °C |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.722 |
| LogP | 3.95 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 24 |
| Complexity | 502 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | C1OC2=CC3C4CC5=C(CN4CCC=3C=C2O1)C1OCOC=1C=C5 |
| InChi Key | UXYJCYXWJGAKQY-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C19H17NO4/c1-2-16-19(24-10-21-16)14-8-20-4-3-12-6-17-18(23-9-22-17)7-13(12)15(20)5-11(1)14/h1-2,6-7,15H,3-5,8-10H2 |
| Chemical Name | 5,7,17,19-tetraoxa-13-azahexacyclo[11.11.0.02,10.04,8.015,23.016,20]tetracosa-2,4(8),9,15(23),16(20),21-hexaene |
| Synonyms | Tetrahydrocoptisine; 4312-32-7; Stylopine; (-)-STYLOPINE; dl-Stylopine; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Natural alkaloid; anti-inflammatory; anti-parasitic |
| ln Vitro | Tetrahydrocoptisine (THC) significantly inhibited LPS-induced TNF-α, interleukin-6(IL-6) and nitric oxide (NO) production. THC inhibited the production of TNF-α and IL-6 by down-regulating LPS-induced IL-6 and TNF-α mRNA expression. Furthermore, it attenuated the phosphorylation of p38 mitogen-activated protein kinase (p38MAPK) and phosphorylation of extracellular signal-regulated kinase1/2 (ERK1/2) as well as the expression of nuclear factor kappa B(NF-κB), in a concentration-dependent manner. Taken together, our data suggest that THC is an active anti-inflammatory constituent by inhibition of TNF-α, IL-6 and NO production possibly via down-regulation of NF-κB activation, phospho-ERK1/2 and phospho-p38MAPK signal pathways[1]. |
| ln Vivo | The extracts or constituents from Corydalis impatiens are known to have many pharmacological activities. Tetrahydrocoptisine (THC), a protoberberine compound from Corydalis impatiens, was found to possess a potent anti-inflammatory effect in different acute or chronic inflammation model animals. Pretreatment with THC (i.p.) inhibited the paw and ear edema in the carrageenan-induced paw edema assay and xylene-induced ear edema assay, respectively. In the lipopolysaccharide (LPS)-induced systemic inflammation model, THC significantly inhibited serum tumor necrosis factor-alpha (TNF-α) release in mice [1]. |
| Enzyme Assay | Assay of myeloperoxidase in gastric tissue[2] Myeloperoxidase, an enzyme found primarily in neutrophil azurophilic granules, has been used extensively as a biochemical marker for granulocyte infiltration into various tissues, including the gastrointestinal tract (Costa et al., 2013, Krawisz et al., 1984). MPO activity was determined using an MPO activity measurement kit by adding 0.2 ml of o-dianisidine hydrochloride and 0.0005% hydrogen peroxide to 4 ml buffer containing 0.2 ml homogenates. MPO activity was assayed at room temperature by measuring the increase in absorbance at 460 nm due to the fluorescent product oxidized by the H2O2-generated redox intermediate. MPO activities were expressed as units per gram of tissue. |
| Animal Protocol |
Ethanol-induced gastric mucosal damage[2]
Mice were randomly divided into five experimental groups, each containing ten animals. The normal and ulcer control groups received vehicle (0.9% saline) throughout the course of the experiments. The prevention groups received (ip) different doses of THC (10 and 20 mg/kg, dissolved in 0.9% saline) and cimetidine (100 mg/kg, reference drug, dissolved in 0.9% saline) respectively for a period of 3 days. After fasting for 24 h prior to the experiment, mice were fed orally with 75% ethanol (0.5 ml/100 g body weight) to induce the acute ulcer, while the normal group received water only (Mei et al., 2012). Four hours after induction, blood samples were collected from the retro-orbital plexus of each animal and were then centrifuged for 10 min at 2500 g to obtain clear sera which were stored at − 80 °C before use (Choi et al., 2010). After the mice were euthanized, the stomachs were rapidly removed, opened along the greater curvature and rinsed with ice-cold saline to remove the gastric contents and blood clots in order to assess the extent of gastric damage. Thereafter, each stomach was dichotomised, with one moiety of stomach immersed in 10% formaldehyde for histological evaluation and gastric tissue from the other moiety stored at − 80 °C for biochemical determinations.[2] Determination of gastric ulcer index[2] The degree of gastric mucosal damage was evaluated from digital pictures, and rated for gross pathology according to the ulcer score scales as previously described (Salga et al., 2012). The lesions were scored as follows: 0: no lesions; 0.5: slight hyperemia or ≤ 5 petechiae; 1: ≤ 5 erosions ≤ 5 mm in length; 1.5: ≤ 5 erosions ≤ 5 mm in length and many petechiae; 2: 6–10 erosions ≤ 5 mm in length; 2.5: 1–5 erosions > 5 mm in length; 3: 5–10 erosions > 5 mm in length; 3.5: > 10 erosions > 5 mm in length; 4: 1–3 erosions ≤ 5 mm in length and 0.5–1 mm in width; 4.5: 4–5 erosions ≤ 5 mm in length and 0.5–1 mm in width; 5: 1–3 erosions > 5 mm in length and 0.5–1 mm in width; 6: 4 or 5 grade 5 lesions; and 7: ≥ 6 grade 5 lesions; 8: complete lesion of the mucosa with hemorrhage.. The sum of the total scores was divided by the number of animals to obtain the mean ulcer index for each group. |
| References |
[1].Anti-inflammatory effect of tetrahydrocoptisine from Corydalis impatiens is a function of possible inhibition of TNF-\u03b1, IL-6 and NO production in lipopolysaccharide-stimulated peritoneal macrophages through inhibiting NF-\u03baB activation and and MAPK pathway. Eur J Pharmacol . 2013 Sep 5;715(1-3):62-71 [2].Protective effect of tetrahydrocoptisine against ethanol-induced gastric ulcer in mice. Toxicol Appl Pharmacol. 2013 Oct 1;272(1):21-9. [3].Isoquinoline alkaloids as a novel type of AKR1C3 inhibitors. J Steroid Biochem Mol Biol . 2014 Sep;143:250-8. |
| Additional Infomation |
Stylopine has been reported in Fibraurea recisa, Corydalis ternata, and other organisms with data available. See also: Stylopine (annotation moved to). |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0927 mL | 15.4636 mL | 30.9272 mL | |
| 5 mM | 0.6185 mL | 3.0927 mL | 6.1854 mL | |
| 10 mM | 0.3093 mL | 1.5464 mL | 3.0927 mL |