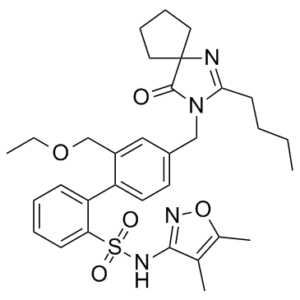
Sparsentan (formerly known as PS433540; BMS-346567; RE-021; DARA-a; Filspari) is a novel, highly potent dual antagonist of angiotensin II and endothelin A receptor for the treatment of IgA nephropathy (IgAN). It inhibits the receptors for endothelin A and angiotensin II at concentrations of 0.8 and 9.3 nM, respectively. More and for a longer period of time than DARA 3 or AT(1) or ET(A) receptor antagonists alone, DARA 7 decreased blood pressure elevations in rats brought on by intravenous infusion of Ang II or big ET-1. The combination of AT(1) and ET(A) receptor blockade in a single molecule was demonstrated by Compound 7, which outperformed irbesartan (an AT(1) receptor antagonist) in the normal SHR model of hypertension in a dose-dependent manner. Approved in 2023 by FDA for treating Proteinuria in primary IgA nephropathy.
Physicochemical Properties
| Molecular Formula | C32H40N4O5S |
| Molecular Weight | 592.7488 |
| Exact Mass | 592.272 |
| Elemental Analysis | C, 64.84; H, 6.80; N, 9.45; O, 13.50; S, 5.41 |
| CAS # | 254740-64-2 |
| Related CAS # | Sparsentan-d5; 1801597-09-0 |
| PubChem CID | 10257882 |
| Appearance | White to off-white solid powder |
| LogP | 7.066 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 42 |
| Complexity | 1060 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=S(C1=CC=CC=C1C2=CC=C(CN3C(CCCC)=NC4(CCCC4)C3=O)C=C2COCC)(NC5=NOC(C)=C5C)=O |
| InChi Key | WRFHGDPIDHPWIQ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35) |
| Chemical Name | 2-[4-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-2-(ethoxymethyl)phenyl]-N-(4,5-dimethyl-1,2-oxazol-3-yl)benzenesulfonamide |
| Synonyms | RE-021; BMS 346567; RE021; Filspari; PS-433540; BMS346567; RE 021; PS 433540; DARA-a; BMS-346567 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Human angiotensin II ( Ki = 0.8 nM ); Human endothelin A ( Ki = 9.3 nM ); Rat angiotensin II ( Ki = 0.4 nM ) | |
| ln Vitro | Sparsentan dose-dependently represses the angiotensin II-induced pressor response with an ED50 value of 0.8 µmol/kg iv and 3.6 µmol/kg po. Sparsentan also demonstrates long-acting and efficacious properties in the large ET-1-induced pressor model. In spontaneously hypertensive rats, sparsentan significantly lowers blood pressure at the lowest dose tested (10 µmol/kg/day). Sparsentan shows good oral bioavailability in rats, dogs, and monkeys, averaging 40%, 86%, and 21% F, respectively. During the course of the drug's pharmacokinetic duration, Sparsentan lowers blood pressure from 170 to less than 100 mmHg at 100 µmol/kg/day. Over the course of its pharmacokinetic duration, sparsentan at 100 µmol/kg/day effectively transforms spontaneously hypertensive rats into normotensive rats[1]. | |
| ln Vivo |
|
|
| Animal Protocol | Rats: The first intravenous bolus injection of angiotensin II was administered to the rats as a control pressor response, following their gavage with vehicle. Angiotensin II is given to the rats at different intervals for a maximum of 240 minutes after irbesartan (30 µmol/kg) and sparsentan (30 µmol/kg) are administered orally (po). Every medication dosage involves 6–8 rats. Angiotensin II pressor effect inhibition is expressed as a percentage (%) based on the difference between the maximum blood pressure increase observed before and after the drug [1]. | |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Following administration of single doses of 200-1600 mg, the Cmax and AUC of sparsentan increase in a less than dose-proportional manner. Sparsentan has time-dependent pharmacokinetics, possibly due to it inducing its own metabolism over time, and at the approved recommended dosage, it reaches steady-state plasma levels within 7 days. Following a single oral dose of 400 mg, the Cmax, AUC and median time to peak plasma concentration of sparsentan are 6.97 μg/mL, 83 μg×h/mL, and 3 hours, respectively. Following daily doses of 400 mg sparsentan, the steady-state Cmax is 6.47 μg/mL, and the AUC is 63.6 μg×h/mL. The administration of a single oral dose (800 mg) of sparsentan with a high-fat, high-calorie meal (1000 kcal, 50% fat) increased the AUC and Cmax by 22% and 108%, respectively. With a single 200 mg dose, a high-fat, high-calorie meal did not have a clinically significant effect on sparsentan pharmacokinetics. Sparsentan is mainly excreted through feces and urine. In healthy subjects given a single dose (400 mg) of radiolabeled sparsentan, approximately 80% of the dose was recovered in feces (9% unchanged) and 2% in urine (negligible amount unchanged). Within a 10-day collection period, 82% of the dosed radioactivity was recovered. At the approved recommended dosage, sparsentan has an apparent volume of distribution at steady state of 61.4 L. Sparsentan has a time-dependent clearance, possibly due to it inducing its own metabolism over time. Following the initial 400 mg dose, sparsentan has an apparent clearance of 3.88 L/h. At steady state, the apparent clearance increases to 5.11 L/h. Metabolism / Metabolites Sparsentan is mainly metabolized by cytochrome P450 3A. Biological Half-Life Sparsentan has an estimated half-life of 9.6 hours at steady state. |
|
| Toxicity/Toxicokinetics |
Protein Binding Sparsentan is more than 99% bound to human plasma proteins. |
|
| References |
[1]. Dual angiotensin II and endothelin A receptor antagonists: synthesis of 2'-substituted N-3-isoxazolyl biphenylsulfonamides with improved potency and pharmacokinetics. J Med Chem. 2005 Jan 13;48(1):171-9. |
|
| Additional Infomation |
Sparsentan is a biphenyl that is 1,1'-biphenyl substituted by (4,5-dimethyl-1,2-oxazol-3-yl)aminosulfonyl, ethoxymethyl, and (2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl groups at positions 2, 2' and 4', respectively. It is a dual antagonist of endothelin and angiotensin II receptors approved for the reduction of proteinuria in adults with primary immunoglobulin A nephropathy at risk of rapid disease progression. It has a role as an angiotensin receptor antagonist, an antihypertensive agent, an endothelin A receptor antagonist and a nephroprotective agent. It is an azaspiro compound, a member of biphenyls, a sulfonamide, a member of isoxazoles and a benzyl ether. Sparsentan is a dual antagonist of the endothelin type A receptor (ETAR) and the angiotensin II (Ang II) type 1 receptor (AT1R) with a similar affinity for both (9.3 nM for ETAR and 0.8 nM for AT1R). Sparsentan is first in its class and orally active, and was created by merging the structural elements of [irbesartan], an AT1R antagonist, and biphenylsulfonamide, an ETAR antagonist. In February 2023, the use of sparsentan to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk of rapid disease progression was approved by the FDA under accelerated approval based on reduction of proteinuria. Sparsentan was initially developed for the treatment of hypertension; however, it has shown to be efficient in the reduction of proteinuria in patients with IgAN and focal segmental glomerulosclerosis (FSGS). Compared to [irbesartan], sparsentan reduces proteinuria to a greater extent. Furthermore, it is the first non-immunosuppressive therapy for the reduction of proteinuria in IgAN. The use of sparsentan may cause hepatotoxicity and embryo-fetal toxicity. Sparsentan is an Endothelin Receptor Antagonist and Angiotensin 2 Receptor Blocker. The mechanism of action of sparsentan is as an Endothelin Receptor Antagonist and Angiotensin 2 Type 1 Receptor Antagonist and Cytochrome P450 2B6 Inducer and Cytochrome P450 2C9 Inducer and Cytochrome P450 2C19 Inducer and P-Glycoprotein Inhibitor and Breast Cancer Resistance Protein Inhibitor. Drug Indication Sparsentan is indicated to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk of rapid disease progression, generally a urine protein-to-creatinine ratio (UPCR) ≥1.5 g/g. Treatment of focal segmental glomerulosclerosis Treatment of primary IgA nephropathy Mechanism of Action Sparsentan is a molecule that acts as a dual antagonist of the endothelin type A receptor (ETAR) and the angiotensin II (Ang II) type 1 receptor (AT1R). It possesses two clinically validated mechanisms of action and selectively blocks the action of two potent vasoconstrictor and mitogenic agents, Ang II and endothelin 1 (ET-1), at their respective receptors. ET-1 and Ang II contribute to the pathogenesis of immunoglobulin A nephropathy (IgAN), a condition characterized by the increased production of galactose-deficient IgA1 (Gd-IgA1) antibodies. Gd-IgA1 antibodies lead to mesangial cell activation and proliferation, which stimulates and is stimulated by ET-1 and Ang II production. The pathological cycle of IgAN results in a compromised glomerular filtration barrier and subsequent proteinuria and haematuria. By acting as both an angiotensin receptor blocker (ARB) and an endothelin receptor antagonist (ERA), sparsentan reduces proteinuria in patients with IgAN. Sparsentan has a high affinity for both ETAR (Ki= 12.8 nM) and AT1R (Ki=0.36 nM), and greater than 500-fold selectivity for these receptors over the endothelin type B and angiotensin II subtype 2 receptors. Pharmacodynamics Sparsentan is a dual endothelin and angiotensin II receptor antagonist. At week 36, the exposure-response (E-R) relationship between sparsentan exposure and the percentage reduction from baseline in urine protein-to-creatinine ratio (UPCR) was not statistically significant over the observed sparsentan exposure range. E-R relationships were not statistically significant for any grade of hypotension or the worst grade of peripheral edema. In healthy subjects, sparsentan caused QTcF prolongation with a maximal mean effect of 8.8 msec at 800 mg and 8.1 msec at 1600 mg. The mechanism behind the observed QTc prolongation is unknown but is unlikely to be mediated via direct inhibition of hERG channels. At the recommended dose, no clinically relevant QTc prolongation is expected. The use of sparsentan may cause hepatotoxicity, embryo-fetal toxicity, hypotension, acute kidney injury, hyperkalemia, and fluid retention. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ~100 mg/mL (~168.7 mM) Ethanol: ~40 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.51 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.08 mg/mL (3.51 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.51 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6871 mL | 8.4353 mL | 16.8705 mL | |
| 5 mM | 0.3374 mL | 1.6871 mL | 3.3741 mL | |
| 10 mM | 0.1687 mL | 0.8435 mL | 1.6871 mL |