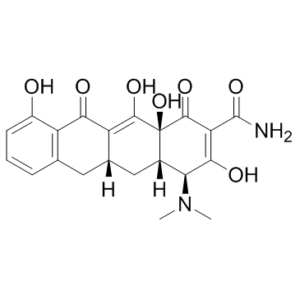
Sancycline (formerly known as GS-2147) is a semi-synthetic tetracycline antibiotic that can be prepared by hydrogenolysis of the chloro and benzylic hydroxy moieties of declomycin. Similar to other tetracyclines, sancycline acts by binding reversibly to the 30S ribosomal subunit and inhibiting protein translation by blocking entry of aminoacyl-tRNA into the ribosome A site. It was first reported in 1962 and is more active than tetracycline against 339 strains of anaerobic bacteria (average MIC90s = 1 and 32 μg/ml, respectively). As the simplest of the early tetracyclines, sancycline was the first to be totally synthesised by Conover and co-workers. Like other tetracyclines, sancycline acts by reversibly binding to the 30S ribosomal subunit and inhibiting protein translation by blocking entry of aminoacyl-tRNA into the ribosome A site.
Physicochemical Properties
| Molecular Formula | C21H22N2O7 | |
| Molecular Weight | 414.41 | |
| Exact Mass | 414.142 | |
| Elemental Analysis | C, 60.86; H, 5.35; N, 6.76; O, 27.02 | |
| CAS # | 808-26-4 | |
| Related CAS # | 6625-20-3 (HCl);808-26-4; | |
| PubChem CID | 54688686 | |
| Appearance | Solid powder | |
| Density | 1.6±0.1 g/cm3 | |
| Boiling Point | 750.9±60.0 °C at 760 mmHg | |
| Melting Point | 224-228ºC (dec) | |
| Flash Point | 407.9±32.9 °C | |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C | |
| Index of Refraction | 1.735 | |
| LogP | -0.67 | |
| Hydrogen Bond Donor Count | 5 | |
| Hydrogen Bond Acceptor Count | 8 | |
| Rotatable Bond Count | 2 | |
| Heavy Atom Count | 30 | |
| Complexity | 892 | |
| Defined Atom Stereocenter Count | 4 | |
| SMILES | O[C@]12C(O)=C3C(C4C(O)=CC=CC=4C[C@H]3C[C@H]1[C@@H](C(=C(C2=O)C(=O)N)O)N(C)C)=O |
|
| InChi Key | XDVCLKFLRAWGIT-ADOAZJKMSA-N | |
| InChi Code | InChI=1S/C21H22N2O7/c1-23(2)15-10-7-9-6-8-4-3-5-11(24)12(8)16(25)13(9)18(27)21(10,30)19(28)14(17(15)26)20(22)29/h3-5,9-10,15,24,26-27,30H,6-7H2,1-2H3,(H2,22,29)/t9-,10-,15-,21-/m0/s1 | |
| Chemical Name | (4S,4aS,5aR,12aS)-4-(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | 30S ribosomal subunit |
| ln Vitro | Sancycline exhibits higher activity than tetracycline against a broad spectrum of anaerobic bacteria, with particularly low MIC values (1 and 32 μg/ml, respectively). It also remains effective against tetracycline-resistant E. coli, S. aureus, and E. faecalis strains, with MICs ranging from 0.06 to 1 μg/ml. In vivo, sancycline demonstrates efficacy against S. aureus in mice, with ED50 values of 0.46 and 0.6 mg/kg for intravenous and subcutaneous administration, respectively. |
| Enzyme Assay | Mushroom tyrosinase activity 80 µL of compounds prepared using 50 mM phosphate buffer (pH 6.5) were aliquoted in a 96-well plate with 100 µL of 0.75 mM L-DOPA. Kojic acid (0.5 mM) was used as a positive control. The reaction was initiated by adding 20 µL of 35 µg/mL solution of mushroom tyrosinase. Dopachrome production was monitored by measuring absorbance at 475 nm (every 30 s for an interval of 10 min). The slopes of the kinetic readings were calculated to determine and compare tyrosinase activity from control [2]. |
| Cell Assay |
Cytotoxicity assay Mitochondria of living cells can convert the tetrazolium salt of the MTS reagent (5-(3-carboxymethoxyphenyl)-2-(4,5-dimethyl-thiazoly)-3-(4-sulfophenyl) tetrazolium salt) into a purple colored formazan product which can be detected spectrophotometrically at 490 nm; the amount of formazan produced is proportional to the viable cell numbers in the culture and can be used as a marker for cell viability. B16F10 cells (5 × 103 cells/well) were seeded in a 96-well plate for 24 h after which the compounds were added in DMSO (final DMSO concentration was 0.4%) while control group consisted of cells treated with 0.4% DMSO; this concentration does not cause any cytotoxicity to either B16F10 or DP cells (Fig. S1), and cultures were maintained for another 72 h. At the end of 72 h, culture medium was replaced by 100 μL of fresh medium with 20 μL of MTS reagent, incubated for 40 min and absorbance was read at 490 nm using a Versamax® microplate reader. Cell viability was calculated from the absorbance values relative to control groups and expressed in %. DP cells (2 × 104 cells/well) were seeded in a 96-well plate for 48 h, followed by replacement of medium with compounds and cultures maintained for 72 h. Viability was measured with an MTS assay as described earlier [2]. |
| References |
[1]. Int J Pharm.2013 Jun 25;450(1-2):225-34. [2]. Arch Dermatol Res. 2023 Mar;315(2):249-257. |
| Additional Infomation | Sancycline is a member of tetracyclines. |
Solubility Data
| Solubility (In Vitro) | DMSO : 8.33~83 mg/mL ( 20.10~200.28 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4131 mL | 12.0653 mL | 24.1307 mL | |
| 5 mM | 0.4826 mL | 2.4131 mL | 4.8261 mL | |
| 10 mM | 0.2413 mL | 1.2065 mL | 2.4131 mL |