SR9009 (SR-9009; Stenabolic) is a novel and potent synthetic REV-ERBα/β agonist with the potential to be used for sleep disorders. It activates REV-ERBα and REV-ERBβ with IC50 values of 670 nM and 800 nM, respectively. Synchronizing rhythms of behaviour and metabolic processes is important for cardiovascular health and preventing metabolic diseases. The nuclear receptors REV-ERB-α and REV-ERB-β have an integral role in regulating the expression of core clock proteins driving rhythms in activity and metabolism. Administration of synthetic REV-ERB ligands alters circadian behaviour and the circadian pattern of core clock gene expression in the hypothalami of mice. The circadian pattern of expression of an array of metabolic genes in the liver, skeletal muscle and adipose tissue was also altered, resulting in increased energy expenditure. Treatment of diet-induced obese mice with a REV-ERB agonist decreased obesity by reducing fat mass and markedly improving dyslipidaemia and hyperglycaemia. These results indicate that synthetic REV-ERB ligands that pharmacologically target the circadian rhythm may be beneficial in the treatment of sleep disorders as well as metabolic diseases.
Physicochemical Properties
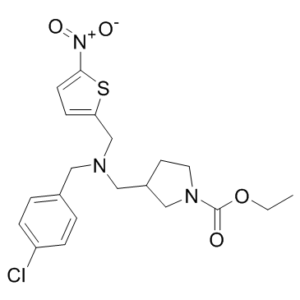
| Molecular Formula | C20H24CLN3O4S | |
| Molecular Weight | 437.94 | |
| Exact Mass | 437.118 | |
| CAS # | 1379686-30-2 | |
| Related CAS # |
|
|
| PubChem CID | 57394020 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 547.2±45.0 °C at 760 mmHg | |
| Flash Point | 284.7±28.7 °C | |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C | |
| Index of Refraction | 1.608 | |
| LogP | 4.17 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 8 | |
| Heavy Atom Count | 29 | |
| Complexity | 556 | |
| Defined Atom Stereocenter Count | 0 | |
| InChi Key | MMJJNHOIVCGAAP-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C20H24ClN3O4S/c1-2-28-20(25)23-10-9-16(13-23)12-22(11-15-3-5-17(21)6-4-15)14-18-7-8-19(29-18)24(26)27/h3-8,16H,2,9-14H2,1H3 | |
| Chemical Name |
|
|
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets |
|
|
| ln Vitro | In a dose-dependent manner, SR9009 promotes the production of the Gal4-responsive luciferase reporter gene and the chimeric Gal4 DNA binding domain (DBD)-REV-ERB ligand binding domain (LBD) α or β (SR9009: REV-ERBα IC50=670 nM, REV-ERBβ IC50=800 nM). In co-transfection tests with full-length REV-ERBα and a luciferase reporter powered by the Bmal1 promoter, SR9009 potently suppresses transcription (IC50=710 nM). In HepG2 cells, SR9009 inhibits BMAL1 mRNA expression in a way that is dependent on REV-ERBα/β. Circular dichroism analysis (Kd=800 nM) was also used to confirm direct binding of SR9009 to REV-ERBα [1]. | |
| ln Vivo | Mice given SR9009 (100 mg/kg, ip) had a more marked decrease in body weight. In addition, plasma glucose (19%) and non-esterified fatty acids (NEFA) were decreased by 23% in rats treated with SR9009. Triglyceride (TG) synthesis-related genes were shown to express less in white adipose tissue (WAT) after treatment with SR9009; this effect was also noted in lean mice [1]. | |
| Enzyme Assay | In this study, researchers developed two REV-ERBα/β agonists with sufficient plasma/brain exposure to allow evaluation of their effects in vivo. Both SR9009 and SR9009 (Fig. 1a, Supplementary Fig. 1) dose-dependently increased the REV-ERB-dependent repressor activity assessed in HEK293 cells expressing a chimeric Gal4 DNA Binding Domain (DBD) - REV-ERB ligand binding domain (LBD) α or β and a Gal4-responsive luciferase reporter (Fig. 1b) (SR9009: REV-ERBα IC50=670 nM, REV-ERBβ IC50=800 nM; SR9011: REV-ERBα IC50=790 nM, REV-ERBβ IC50=560 nM). The REV-ERB ligand GSK4112 (Supplementary Fig. 2), which exhibits no plasma exposure displays limited activity (Fig. 1b). Both SR9011 and SR9009 potently and efficaciously suppressed transcription in a cotransfection assay using full-length REV-ERBα along with a luciferase reporter driven by the Bmal1 promoter (Fig. 1c) (SR9009 IC50=710 nM; SR9011 IC50=620 nM). SR9011 and SR9009 suppressed the expression of BMAL1 mRNA in HepG2 cells in a REV-ERBα/β-dependent manner (Supplementary Fig. 3). Consistent with both compounds functioning as direct agonists of REV-ERB, we noted that the compounds increased the recruitment of the CoRNR box peptide fragment of NCoR using a biochemical assay (Supplementary Fig. 4). Direct binding of the SR9009 to REV-ERBα was also confirmed using circular dichrosim analysis (Supplementary Fig. 5) (Kd=800 nM). Neither compound exhibited activity at other nuclear receptors12,13 (Supplementary Fig. 6)[1]. | |
| Cell Assay |
|
|
| Animal Protocol | For circadian gene expression experiments male C57BL6 mice (8–10 weeks of age) were either maintained on a L:D (12h:12h) cycle or on constant darkness. At circadian time (CT) 0 animals were administered a single dose of 100 mg/kg SR9009 or SR9011 (i.p.) and groups of animals (n=6) were sacrificed at CT0, CT6, CT12 and CT18. Gene expression was determined by real time QPCR.[1] | |
| References |
[1]. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012 Mar 29;485(7396):62-68. |
|
| Additional Infomation |
SR-9009 is a REV-ERB agonist. SR-9011 has been demonstrated that it is specifically lethal to cancer cells and oncogene-induced senescent cells, including melanocytic naevi, and has no effect on the viability of normal cells or tissues. Synchronizing rhythms of behaviour and metabolic processes is important for cardiovascular health and preventing metabolic diseases. The nuclear receptors REV-ERB-α and REV-ERB-β have an integral role in regulating the expression of core clock proteins driving rhythms in activity and metabolism. Here we describe the identification of potent synthetic REV-ERB agonists with in vivo activity. Administration of synthetic REV-ERB ligands alters circadian behaviour and the circadian pattern of core clock gene expression in the hypothalami of mice. The circadian pattern of expression of an array of metabolic genes in the liver, skeletal muscle and adipose tissue was also altered, resulting in increased energy expenditure. Treatment of diet-induced obese mice with a REV-ERB agonist decreased obesity by reducing fat mass and markedly improving dyslipidaemia and hyperglycaemia. These results indicate that synthetic REV-ERB ligands that pharmacologically target the circadian rhythm may be beneficial in the treatment of sleep disorders as well as metabolic diseases.[1] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 20 mg/mL (45.67 mM) in 5% DMSO 10% Cremophor EL + 85% ddH2O (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. Solubility in Formulation 2: 2.5 mg/mL (5.71 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: 2.5 mg/mL (5.71 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 4: 2.08 mg/mL (4.75 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 5: 2.08 mg/mL (4.75 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 6: ≥ 2.08 mg/mL (4.75 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2834 mL | 11.4171 mL | 22.8342 mL | |
| 5 mM | 0.4567 mL | 2.2834 mL | 4.5668 mL | |
| 10 mM | 0.2283 mL | 1.1417 mL | 2.2834 mL |