SMIP004 also known as N-(4-butyl-2-methyl-phenyl) is a novel specific inducer of cancer-cell-specific apoptosis in human prostate cancer cells. Positive cell cycle regulators were downregulated by SMIP004, while cyclin-dependent kinase inhibitors were upregulated. These changes led to G1 arrest, the prevention of soft agar colony formation, and cell death. However, it was still unclear how SMIP004 caused cancer cells to selectively undergo apoptosis. Prostate and breast cancer xenografts in mice are potently inhibited by SMIP004. Our findings indicate that SMIP004 targets specific redox and bioenergetic sensitivities of prostate cancer cells that can be used as a therapeutic target by causing the production of mitochondrial ROS.
Physicochemical Properties
| Molecular Formula | C13H19NO | |
| Molecular Weight | 205.3 | |
| Exact Mass | 205.147 | |
| Elemental Analysis | C, 76.06; H, 9.33; N, 6.82; O, 7.79 | |
| CAS # | 143360-00-3 | |
| Related CAS # |
|
|
| PubChem CID | 2747581 | |
| Appearance | White to off-white solid powder | |
| Density | 1.003g/cm3 | |
| Boiling Point | 343ºC at 760mmHg | |
| Flash Point | 206.1ºC | |
| Vapour Pressure | 7.24E-05mmHg at 25°C | |
| Index of Refraction | 1.539 | |
| LogP | 3.369 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 1 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 15 | |
| Complexity | 203 | |
| Defined Atom Stereocenter Count | 0 | |
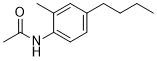
| SMILES | CC1=C(NC(C)=O)C=CC(CCCC)=C1 |
|
| InChi Key | ZFVMECVBUGMWIX-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C13H19NO/c1-4-5-6-12-7-8-13(10(2)9-12)14-11(3)15/h7-9H,4-6H2,1-3H3,(H,14,15) | |
| Chemical Name | N-(4-butyl-2-methylphenyl)acetamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | SKP2 E3 ligase | |
| ln Vitro | SMIP004, a N-(4-butyl-2-methyl-phenyl) acetamide, is a novel specific inducer of cancer-cell selective apoptosis of human prostate cancer cells. SMIP004 decreased the levels of positive cell cycle regulators, upregulated cyclin-dependent kinase inhibitors, and resulted in G1 arrest, inhibition of colony formation in soft agar, and cell death. However, the mechanism of SMIP004-induced cancer cell selective apoptosis remained unknown. SMIP004 potently inhibits the growth of prostate and breast cancer xenografts in mice. Our data suggest that SMIP004, by inducing mitochondrial ROS formation, targets specific sensitivities of prostate cancer cells to redox and bioenergetic imbalances that can be exploited in cancer therapy. | |
| ln Vivo |
|
|
| Enzyme Assay | SMIP004 also known as N-(4-butyl-2-methyl-phenyl) is a novel specific inducer of cancer-cell-specific apoptosis of human prostate cancer cells. | |
| Cell Assay | Whereas SMIP012 and 016 were moderately toxic in normal fibroblasts, SMIPs 001 and 004 showed substantial cancer cell specificity being at least five times more potent in LNCaP-S14 than in IMR90 cells , treatment with either MG132 or SMIP004 increased p27 half-life to > 6 h. SMIP004 also decreased the quantities of cyclins E and A obtained using CDK2. Cyclins E and A significantly decreased after receiving SMIP004 treatment, which was paralleled by this. Positive cell cycle regulators were downregulated by SMIP004, cyclin-dependent kinase inhibitors were upregulated, G1 arrest, soft agar colony formation was inhibited, and cell death occurred. | |
| Animal Protocol | Mice with prostate and breast cancer xenografts | |
| References |
[1]. J Exp Clin Cancer Res. 2019 Feb 13;38(1):76. [2]. Oncotarget. 2013 Aug;4(8):1212-29.. |
|
| Additional Infomation | SMIP004 is a member of the class of N-benzylacetamides that is benzene substituted by acetylnitrilo, methyl, and butyl groups at positions 1, 2 and 4, respectively. It is a SKP2 E3 ligase inhibitor and a selective apoptosis inducer of human prostate cancer cells. It has a role as an antineoplastic agent, an apoptosis inducer, an antidepressant, an EC 6.3.2.19 (ubiquitin--protein ligase) inhibitor, an autophagy inducer and an anticoronaviral agent. It is a member of toluenes, a member of N-benzylacetamides and an alkylbenzene. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (12.18 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (12.18 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8709 mL | 24.3546 mL | 48.7092 mL | |
| 5 mM | 0.9742 mL | 4.8709 mL | 9.7418 mL | |
| 10 mM | 0.4871 mL | 2.4355 mL | 4.8709 mL |