SCH-1473759 (SCH1473759) is a novel, potent and selective aurora inhibitor with anticancer effects. With IC50s of 4 and 13 nM, respectively, it suppresses aurora A and B. SCH 1473759 exhibited efficacy against an extensive array of tumor cell lines originating from diverse tissue origins and genetic backgrounds. SCH-1473759 exhibited strong mechanism-based activity, and when combined with taxanes and KSP inhibitors, activity was found to be amplified. Enhancing the clinical effectiveness of aurora inhibitors may benefit from this knowledge.
Physicochemical Properties
| Molecular Formula | C20H26N8OS |
| Molecular Weight | 426.53844 |
| Exact Mass | 426.195 |
| Elemental Analysis | C, 56.32; H, 6.14; N, 26.27; O, 3.75; S, 7.52 |
| CAS # | 1094069-99-4 |
| Related CAS # | SCH-1473759 hydrochloride;1094067-13-6 |
| PubChem CID | 25144732 |
| Appearance | White to yellow solid powder |
| Density | 1.4±0.1 g/cm3 |
| Index of Refraction | 1.714 |
| LogP | 2.55 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 30 |
| Complexity | 569 |
| Defined Atom Stereocenter Count | 0 |
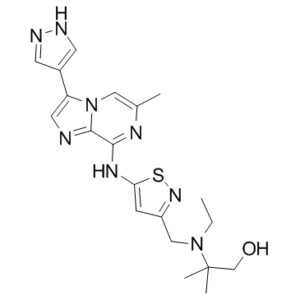
| SMILES | CC1=CN2C(C(NC3=CC(CN(C(C)(CO)C)CC)=NS3)=N1)=NC=C2C4=CNN=C4 |
| InChi Key | RHGZQGXELRMGES-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C20H26N8OS/c1-5-27(20(3,4)12-29)11-15-6-17(30-26-15)25-18-19-21-9-16(14-7-22-23-8-14)28(19)10-13(2)24-18/h6-10,29H,5,11-12H2,1-4H3,(H,22,23)(H,24,25) |
| Chemical Name | 2-[ethyl-[[5-[[6-methyl-3-(1H-pyrazol-4-yl)imidazo[1,2-a]pyrazin-8-yl]amino]-1,2-thiazol-3-yl]methyl]amino]-2-methylpropan-1-ol |
| Synonyms | SCH-1473759; SCH1473759; SCH 1473759 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Aurora A (IC50 = 4 nM); Aurora B (IC50 = 13 nM) |
| ln Vitro | SCH-1473759 exhibits a 20 nM Kd for aurora A and 30 nM Kd for aurora B. SCH-1473759 also inhibits VEGFR2 (IC50=1 nM), IRAK4 (IC50=37 nM), Chk1 (IC50=13 nM), and the Src family of kinases (IC50<10 nM). Against 34 additional kinases that belong to various kinome families, it exhibits no discernible activity (IC50>1000 nM). An IC50 of 6 nM is required for SCH-1473759 to inhibit the proliferation of HCT116 cells[1]. Breast, ovarian, prostate, lung, colon, brain, gastric, renal, skin, and leukemia tumor cell lines are inhibited by SCH 1473759. According to research, A2780, LNCap, N87, Molt4, K562, and CCRF-CEM are among the most sensitive cell lines, with IC50 values less than 5 nM[2]. |
| ln Vivo | SCH-1473759 exhibits 50% tumor growth inhibition (TGI) at a low dose of 5 mg/kg (ip, bid), which is well-tolerated in a continuous dosing schedule on day 16. When administered on an intermittent schedule (5 days on, 5 days off), a higher dose of 10 mg/kg (ip, bid) is well tolerated and results in 69% TGI on day 16. All species exhibit good exposure to SCH-1473759, with a high clearance in rodents and a moderate clearance in dogs and monkeys. Although the tissue distribution is high, the half-life is likewise moderate[1]. Four human tumor xenograft models demonstrated the dose- and schedule-dependent anti-tumor activity of SCH 1473759. Additionally, the efficacy is increased when taxanes are added, and it is discovered that the most effective time to dose SCH 1473759 is 12 hours after taxane treatment[2]. |
| Enzyme Assay | In 384-well plates with low protein binding, the Aurora A and Aurora B kinase assays are run. To obtain the appropriate concentrations, SCH-1473759 is diluted in 100% DMSO. Enzyme Aurora A (8 nM), 100 nM Tamra-PKAtide, 25μM ATP, 1 mM DTT, and kinase buffer are needed for each reaction in the Aurora A assay. Enzyme Aurora B (26 nM), 100 nM Tamra-PKAtide, 50 μM ATP, 1 mM DTT, and kinase buffer were required for each reaction in the Aurora B assay. Inhibition data from 8 point serial dilutions of SCH-1473759 that were generated in duplicate are used to plot dose-response curves[1]. |
| Cell Assay | The cells are treated with SCH-1473759 (0.1% final DMSO concentration) in triplicate wells after being plated at a density of 625 to 3,750 cells per well. At the beginning of the study (zero hour), one plate is stained, and another plate is incubated at 37°C for 72 hours before being stained. Following a 30-minute incubation period, cells are fixed using a fixation solution and 1,000 nM Hoechst 33342 dye. After removing the fixation solution, cells are ished twice using PBS. Next, using an automated fluorescent microscope, 15 immunofluorescence images are taken at a 10X magnification [1]. |
| Animal Protocol | Mice: In mice with established A2780 ovarian tumor xenografts, the antitumor efficacy of SCH 1473759 dosed intraperitoneally is assessed. Ten mg/kg bid (twice daily), twenty mg/kg qd (daily), and one hundred mg/kg day 0, 4, 7 are the three schedules that are tested at their respective maximum tolerated doses. Also tested is 60 mg/kg on days 0, 4, and 7[2]. |
| References |
[1]. Discovery of a Potent, Injectable Inhibitor of Aurora Kinases Based on the Imidazo-[1,2-a]-Pyrazine Core. ACS Med Chem Lett. 2010 Jun 7;1(5):214-8. [2]. SCH 1473759, a novel Aurora inhibitor, demonstrates enhanced anti-tumor activity in combination with taxanes and KSP inhibitors. Cancer Chemother Pharmacol. 2011 Oct;68(4):923-33. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3444 mL | 11.7222 mL | 23.4445 mL | |
| 5 mM | 0.4689 mL | 2.3444 mL | 4.6889 mL | |
| 10 mM | 0.2344 mL | 1.1722 mL | 2.3444 mL |