SB-649868 (GSK-649868) is a novel, potent, selective and orally bioactive orexin (OX) 1 and OX2 receptor antagonist with pKi of 9.4 and 9.5 for OX1 and OX2 receptor, respectively. It might be applied to the treatment of sleep disorders and insomnia. Two G-protein coupled receptors, the OX(1) and OX(2) receptors, are strongly agonistic for the hypothalamic peptides orexin-A and orexin-B. In the rat brain, these receptors are widely dispersed albeit in different ways. The OX(2) receptor is primarily found in the ventral posterior nucleus, whereas the OX(1) receptor is highly expressed throughout the hypothalamus.
Physicochemical Properties
| Molecular Formula | C26H24FN3O3S | |
| Molecular Weight | 477.55 | |
| Exact Mass | 477.152 | |
| Elemental Analysis | C, 65.39; H, 5.07; F, 3.98; N, 8.80; O, 10.05; S, 6.71 | |
| CAS # | 380899-24-1 | |
| Related CAS # |
|
|
| PubChem CID | 25195495 | |
| Appearance | White to off-white solid powder | |
| LogP | 5.941 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 34 | |
| Complexity | 734 | |
| Defined Atom Stereocenter Count | 1 | |
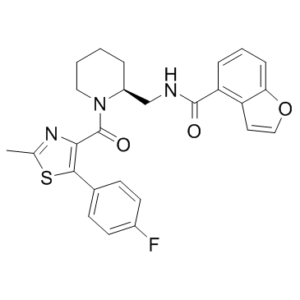
| SMILES | O=C(C1=C(C2=CC=C(F)C=C2)SC(C)=N1)N3CCCC[C@H]3CNC(C4=C(C=CO5)C5=CC=C4)=O |
|
| InChi Key | ZJXIUGNEAIHSBI-IBGZPJMESA-N | |
| InChi Code | InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | |
| Chemical Name | N-[[(2S)-1-[5-(4-fluorophenyl)-2-methyl-1,3-thiazole-4-carbonyl]piperidin-2-yl]methyl]-1-benzofuran-4-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | OX1 ( pKi = 9.4 ); OX2 ( pKi = 9.5 ) |
| ln Vitro | SB-649868 is recognized as one of the most powerful dual OX1 and OX2 receptor antagonists that was previously available in vitro (pKi=9.4 and 9.5 at the OX1 and OX2 receptors, respectively) [1]. With the following pKB value (OX1=9.67; OX2=9.64), SB-649868 inhibits the accumulation of inositol 1 phosphate (IP1) induced by orexin-A. With the following pKi values, OX1=9.27 and OX2=8.91, SB-649868 displaces the [3H]ACT-078573 receptor binding. SB-649868 exhibits a clear non-surmountable behavior as concentrations range from 0.3 nM to 30 nM. This results in a rightward shift of the orexin-A CRCs and a depression of the agonist efficacy. The estimated apparent pKB values for OX1 and OX2 are 9.67±0.03 and 9.64±0.07, respectively[2]. |
| Cell Assay | Chinese Hamster Ovary (CHO) cells are cultured in Dulbecco's modified Eagle's medium F12 Ham, supplemented with 10% fetal bovine serum (FBS), 2 mg/mL glutamine, and 600 μg/ml geneticin. The cells are transfected with the human OX1 orexin receptor and are kept at 37 °C in an environment that is 95% air and 5% CO2. Stable transfected CHO cells expressing the human OX2 orexin receptor are grown in alpha-MEM enhanced with 10% FBS, 100 u/mL penicillin G, 100 u/mL streptomycin, and 400 μg/mL geneticin. The culture is maintained at 37 °C in a 95% air and 5% CO2 environment. With the help of the IP-One HTRF terbium cryptate-based assay, IP1 accumulation is quantified. OX1-CHO cells are cultured for 24 hours in the presence of 5 mM sodium butyrate after being seeded at a density of 1×104 cells per well into a white 384-well plate, whereas OX2-CHO cells are cultured for 24 hours in culture medium. Following a room temperature wash with Hank's Balanced Salt Solution (HBSS) containing 20 mM HEPES pH 7.4, 50 mM LiCl, and 0.1% Bovine Serum Albumin (BSA), cells are incubated with antagonist for 45 minutes before being treated with agonist for 60 minutes at 37 °C. After being diluted in lysis buffer, detection reagents, IP1-d2 tracer, and anti-IP1-cryptate are added to the cells. Envision Multilabel flash lamp reader with 100 flashes and 400 μs integration time is used to measure time-resolved fluorescence at 615 nm and 665 nm after 60 minutes of room temperature incubation[2]. |
| References |
[1]. The Discovery of LML134, a Histamine H3 Receptor Inverse Agonist for the Clinical Treatment of Excessive Sleep Disorders. ChemMedChem. 2019 Jul 3;14(13):1238-1247. |
| Additional Infomation | SB-649868 is under investigation in clinical trial NCT01030939 (Study to Investigate Safety, Tolerability, Pharmacokinetics and Cardiac Function After Repeat Doses of SB-649868 in Healthy-volunteers). |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.24 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.24 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0940 mL | 10.4701 mL | 20.9402 mL | |
| 5 mM | 0.4188 mL | 2.0940 mL | 4.1880 mL | |
| 10 mM | 0.2094 mL | 1.0470 mL | 2.0940 mL |