Physicochemical Properties
| Molecular Formula | C21H19N5O4S |
| Molecular Weight | 437.471662759781 |
| Exact Mass | 437.115 |
| CAS # | 1016543-77-3 |
| Related CAS # | Valecobulin;1188371-47-2;Valecobulin hydrochloride;1240321-53-2 |
| PubChem CID | 46929538 |
| Appearance | Light yellow to yellow solid powder |
| LogP | 3.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 31 |
| Complexity | 602 |
| Defined Atom Stereocenter Count | 0 |
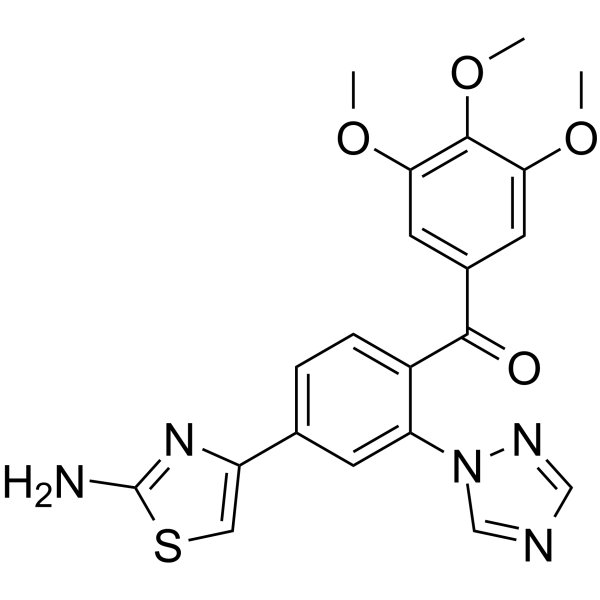
| SMILES | S1C(N)=NC(=C1)C1=CC=C(C(C2C=C(C(=C(C=2)OC)OC)OC)=O)C(=C1)N1C=NC=N1 |
| InChi Key | OJZSPKKXYGZDRQ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C21H19N5O4S/c1-28-17-7-13(8-18(29-2)20(17)30-3)19(27)14-5-4-12(15-9-31-21(22)25-15)6-16(14)26-11-23-10-24-26/h4-11H,1-3H3,(H2,22,25) |
| Chemical Name | [4-(2-amino-1,3-thiazol-4-yl)-2-(1,2,4-triazol-1-yl)phenyl]-(3,4,5-trimethoxyphenyl)methanone |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | IC50: 4.29 μM (tubulin polymerization)[1] |
| ln Vitro | S516 exhibits strong cytotoxicity against HL-60, HCT116, and HCT15 cells, with IC50s of 4.8 nM, 42.8 nM, and 24.9 nM, respectively[1]. S516 (Compound 22; 30 nM; 16 hours; HL60 cells) treatment results in a considerable arrest of cells at the G2/M phase, which is followed by apoptosis and the loss of the G0/G1 phase simultaneously[1]. |
| ln Vivo | In human LX-1 lung cancer and CX-1 colon cancer mouse xenografts, S516 (Compound 22; 5–10 mg/kg; intraperitoneal injection; mice) therapy exhibits encouraging anticancer activity (inhibition ratio (IR)> 63%)[1]. |
| Cell Assay |
Cell Cycle Analysis[1] Cell Types: HL60 cells Tested Concentrations: 30 nM Incubation Duration: 16 hrs (hours) Experimental Results: Caused significant arrest of cells at the G2/M phase, resulting in apoptosis with concomitant loss of G0/G1 phase. |
| Animal Protocol |
Animal/Disease Models: Mice bearing 3LL lung cancer[1] Doses: 5 mg/kg, 10 mg/kg Route of Administration: intraperitoneal (ip)injection Experimental Results: Had promising antitumor activity (inhibition ratio (IR)> 63%). |
| References |
[1]. Identification of CKD-516: a potent tubulin polymerization inhibitor with marked antitumor activity against murine and human solid tumors. J Med Chem. 2010 Sep 9;53(17):6337-54. |
Solubility Data
| Solubility (In Vitro) | DMSO : 12.5 mg/mL (28.57 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.25 mg/mL (2.86 mM) (saturation unknown) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 12.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 + to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2859 mL | 11.4294 mL | 22.8587 mL | |
| 5 mM | 0.4572 mL | 2.2859 mL | 4.5717 mL | |
| 10 mM | 0.2286 mL | 1.1429 mL | 2.2859 mL |