RO-3306 (RO3306; RO 3306) is a novel, potent, ATP-competitive, and selective CDK1 inhibitor with potential antineoplastic activity. It has a Ki of 20 nM for CDK1 inhibition and >15-fold selectivity against several closely related human kinases. RO-3306 treatment of growing AML cells resulted in dose- and time-dependent G2/M-phase cell cycle arrest and apoptosis. RO-3306 inhibited p53-mediated induction of p21 and MDM2, and downregulated the expression of the antiapoptotic proteins Bcl-2 and survivin. In order to encourage apoptosis, RO-3306 actively amplifies downstream p53 signaling.
Physicochemical Properties
| Molecular Formula | C18H13N3OS2 | |
| Molecular Weight | 351.45 | |
| Exact Mass | 351.049 | |
| Elemental Analysis | C, 61.52; H, 3.73; N, 11.96; O, 4.55; S, 18.24 | |
| CAS # | 872573-93-8 | |
| Related CAS # |
|
|
| PubChem CID | 135400873 | |
| Appearance | Yellow to brown solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 569.1±60.0 °C at 760 mmHg | |
| Flash Point | 298.0±32.9 °C | |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C | |
| Index of Refraction | 1.746 | |
| LogP | 3.24 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 24 | |
| Complexity | 548 | |
| Defined Atom Stereocenter Count | 0 | |
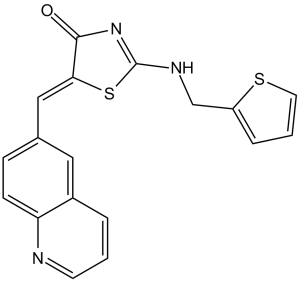
| SMILES | C(=C1/C(=O)N=C(NCC2SC=CC=2)S/1)\C1C=CC2N=CC=CC=2C=1 |
|
| InChi Key | XOLMRFUGOINFDQ-YBEGLDIGSA-N | |
| InChi Code | InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22)/b16-10- | |
| Chemical Name | (5Z)-5-(quinolin-6-ylmethylidene)-2-(thiophen-2-ylmethylimino)-1,3-thiazolidin-4-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CDK1 (Ki = 20 nM); CDK1/cyclinB1 (Ki = 35 nM); CDK1/cyclin A (Ki = 110 nM); CDK2/cyclinE (Ki = 340 nM); PKCδ (Ki = 318 nM); SGK (Ki = 497 nM); ERK (Ki = 1980 nM) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | Recombinant human CDK/cyclin complexes—CDK1/cyclin B1, CDK1/cyclin A, CDK2/cyclin E, and CDK4/cyclin D—expressed and separated from Hi5 insect cells are used in the CDK assays. The assay makes use of GST-cyclin B1, CDK1, GST-cyclin-E, CDK2, GST-CDK4, and cyclin D. Together with their partners, the GST-tagged proteins are coexpressed and purified in complex. A His-6-tagged portion of pRB (amino acids 385–928) is used as the substrate in all assays. From a construct, the protein is expressed. The protein is expressed in M15 Escherichia coli cells, bound to an agarose column that has been Ni-chalated, pretreated with 1 mM imidazole, and then eluted with 500 mM imidazole. Following an aliquoting process and dialyzation against 20 mM Hepes at pH 7/6.25 mM MgCl2/1.5 mM DTT, the eluted protein is kept at -80°C. | |
| Cell Assay | In 96-well plates, log phase cells (25,000) are seeded and CO2 is added to an incubator set at 37°C. In order to ascertain the drug concentrations necessary to attain a 50% growth inhibition (IC50), various doses of RO-3306 are given after a 24-hour period. The cells are incubated for 4 hours after MTT (20 μL, 5 mg/mL stock solution in saline) is added to each well. After the removal of supernatants, 200 μL of anhydrous DMSO is used to dissolve the formazan crystals from viable cells. 565 nm is the wavelength at which the absorbance is measured using a microplate reader model 550. | |
| Animal Protocol |
Female BALB/c mice 1.5 mg/kg i.n. |
|
| References |
[1]. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10660-5. [2]. Cyclin-dependent kinase 1 inhibitor RO-3306 enhances p53-mediated Bax activation and mitochondrial apoptosis in AML. Cancer Sci. 2009 Jun;100(6):1128-36. [3]. A specific inhibitor of CDK1, RO-3306, reversibly arrests meiosis during in vitro maturation of porcine oocytes. Anim Reprod Sci. 2014 Jan 30;144(3-4):102-8. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.67 mg/mL (4.75 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.67 mg/mL (4.75 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: 5 mg/mL (14.23 mM) in 0.5% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8454 mL | 14.2268 mL | 28.4535 mL | |
| 5 mM | 0.5691 mL | 2.8454 mL | 5.6907 mL | |
| 10 mM | 0.2845 mL | 1.4227 mL | 2.8454 mL |