Ribocil-C is a novel, potenty and highly selective chemical modulator of bacterial riboflavin riboswitches. Ribocil-C strongly inhibits GFP expression, achieving a 50% effective concentration (EC50) of 0.3 μM. Ribocil-C is a highly specific bioactive synthetic mimic of FMN, which competes with the natural ligand to inhibit FMN riboswitch-mediated expression of ribB and inhibits bacterial growth. Riboswitches are non-coding RNA structures located in messenger RNAs that bind endogenous ligands, such as a specific metabolite or ion, to regulate gene expression. As such, riboswitches serve as a novel, yet largely unexploited, class of emerging drug targets. Demonstrating this potential, however, has proven difficult and is restricted to structurally similar antimetabolites and semi-synthetic analogues of their cognate ligand, thus greatly restricting the chemical space and selectivity sought for such inhibitors.
Physicochemical Properties
| Molecular Formula | C21H21N7OS |
| Molecular Weight | 419.5027 |
| Exact Mass | 419.152 |
| Elemental Analysis | C, 60.13; H, 5.05; N, 23.37; O, 3.81; S, 7.64 |
| CAS # | 1825355-56-3 |
| Related CAS # | Ribocil;1381289-58-2;Ribocil-C (R enantiomer);2177266-81-6;Ribocil B;1825355-55-2;Ribocil-C Racemate;2309762-18-1 |
| PubChem CID | 136981150 |
| Appearance | Solid powder |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 672.0±65.0 °C at 760 mmHg |
| Flash Point | 360.2±34.3 °C |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.771 |
| LogP | 1.25 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 30 |
| Complexity | 693 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | S1C([H])=C([H])C([H])=C1C1=C([H])C(N([H])C(C2([H])C([H])([H])N(C([H])([H])C3=C([H])N(C4N=C([H])C([H])=C([H])N=4)C([H])=N3)C([H])([H])C([H])([H])C2([H])[H])=N1)=O |
| InChi Key | UVDVCDUBJWYRJW-HNNXBMFYSA-N |
| InChi Code | InChI=1S/C21H21N7OS/c29-19-10-17(18-5-2-9-30-18)25-20(26-19)15-4-1-8-27(11-15)12-16-13-28(14-24-16)21-22-6-3-7-23-21/h2-3,5-7,9-10,13-15H,1,4,8,11-12H2,(H,25,26,29)/t15-/m0/s1 |
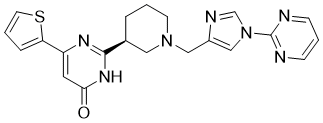
| Chemical Name | 2-[(3S)-1-[[1-(2-Pyrimidinyl)-1H-imidazol-4-yl]methyl]-3-piperidinyl]-6-(2-thienyl)-4(3H)-pyrimidinone |
| Synonyms | Ribocil-C |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Bacterial riboflavin riboswitches |
| ln Vitro | The flavin mononucleotide (FMN) riboswitch, which regulates the expression of de novo riboflavin (RF, vitamin B2) biosynthesis in Escherichia coli, is highly selectively inhibited by ribocil-C. In order to independently regulate the RF biosynthesis and uptake processes necessary for Staphylococcus aureus growth and pathogenesis, riboflavin-C specifically inhibits dual FMN riboswitches[1]. In order to inhibit ribB expression and RF synthesis, which in turn stops bacterial growth, Ribocil-C, a synthetic small-molecule FMN mimic, binds the FMN riboswitch of several GN bacteria, such as Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii[1][2]. |
| ln Vivo | Increased dosage In comparison to sham-treated mice, the ribocil-C treatment groups (60 and 120 mg kg21 ribocil-C) show a dose-dependent reduction in bacterial burden of 1.87 and 3.29 log10[CFU per g spleen] reduction, respectively, without mortality or obvious toxicity effects[2]. |
| Animal Protocol | Intraperitoneal injection of Escherichia coli strain MB5746 (5×104 CFU/mouse) is used to infect DBA/2J mice. The infection is treated with three subcutaneous injections of either ciprofloxacin (0.5 mg/kg) or Ribocil-C (30, 60, 120 mg/kg) over a 24-hour period. Five mice per group have their spleens aseptically removed, and the reduction of log[CFU per g spleen tissue] is computed based on the amount of bacteria present in the spleens of the control group that received a 10% DMSO treatment[2]. |
| References |
[1]. Dual-Targeting Small-Molecule Inhibitors of the Staphylococcus aureus FMN Riboswitch DisruptRiboflavin Homeostasis in an Infectious Setting. Cell Chem Biol. 2017 May 18;24(5):576-588. [2]. Selective small-molecule inhibition of an RNA structural element. Nature. 2015 Oct 29;526(7575):672-7. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~110 mg/mL (~262.22 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.75 mg/mL (6.56 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 27.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.75 mg/mL (6.56 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 27.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 10% DMSO+90% Corn Oil: ≥ 2.75 mg/mL (6.56 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3838 mL | 11.9190 mL | 23.8379 mL | |
| 5 mM | 0.4768 mL | 2.3838 mL | 4.7676 mL | |
| 10 mM | 0.2384 mL | 1.1919 mL | 2.3838 mL |