Physicochemical Properties
| Molecular Formula | C41H44CL3F2N7O3 |
| Molecular Weight | 827.188973426819 |
| Exact Mass | 863.227 |
| CAS # | 2387505-58-8 |
| Related CAS # | RWJ-56110;252889-88-6 |
| PubChem CID | 90488773 |
| Appearance | White to light yellow solid powder |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 15 |
| Heavy Atom Count | 57 |
| Complexity | 1240 |
| Defined Atom Stereocenter Count | 2 |
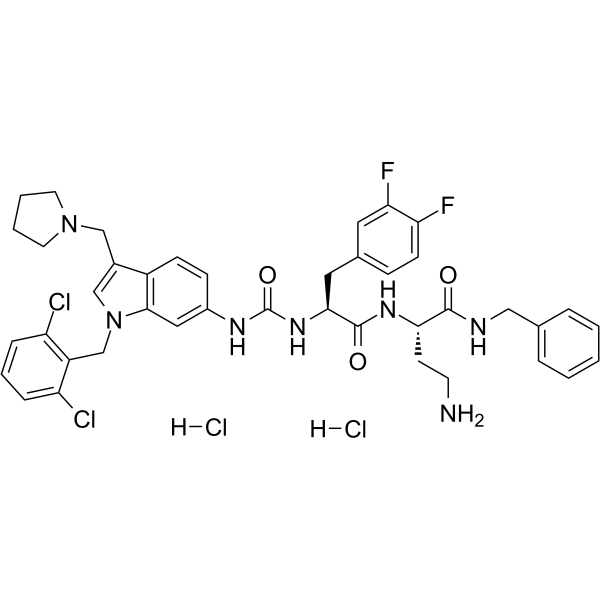
| SMILES | C(C1C(=CC=CC=1Cl)Cl)N1C=C(CN2CCCC2)C2=CC=C(NC(=O)N[C@H](C(=O)N[C@@H](CCN)C(=O)NCC3C=CC=CC=3)CC3C=CC(F)=C(F)C=3)C=C12.Cl |
| InChi Key | MGZSVSHDIOPSBO-ZEUUMAKDSA-N |
| InChi Code | InChI=1S/C41H43Cl2F2N7O3.2ClH/c42-32-9-6-10-33(43)31(32)25-52-24-28(23-51-17-4-5-18-51)30-13-12-29(21-38(30)52)48-41(55)50-37(20-27-11-14-34(44)35(45)19-27)40(54)49-36(15-16-46)39(53)47-22-26-7-2-1-3-8-26;;/h1-3,6-14,19,21,24,36-37H,4-5,15-18,20,22-23,25,46H2,(H,47,53)(H,49,54)(H2,48,50,55);2*1H/t36-,37-;;/m0../s1 |
| Chemical Name | (2S)-4-amino-N-benzyl-2-[[(2S)-2-[[1-[(2,6-dichlorophenyl)methyl]-3-(pyrrolidin-1-ylmethyl)indol-6-yl]carbamoylamino]-3-(3,4-difluorophenyl)propanoyl]amino]butanamide;dihydrochloride |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | IC50: 0.44 uM (PAR-1) IC50: 0.16 μM (the aggregation of human platelets induced by SFLLRN-NH2) IC50: 0.34 μM (the aggregation of human platelets induced by thrombin)[1][2] |
| ln Vitro | The N-terminal extracellular domain of the proteinase-activated receptors (PARs) family of G protein-coupled receptors is cleaved by proteases, revealing a novel amino terminus sequence that acts as a tethered ligand to activate the receptors. When it comes to collagen and the thromboxane mimetic U46619 (HY-108566), RWJ56110 is very selective, but it also inhibits the aggregation of human platelets caused by both SFLLRN-NH2 (IC50=0.16 μM) and thrombin (IC50=0.34 μM).[1]. At an IC50 value of 3.5 μM, RWJ-56110 dihydrochloride completely suppresses the proliferation of RASMCs caused by thrombin. When RWJ-56110 dihydrochloride is used in conjunction with RASMC calcium mobilization (IC50=0.12 μM), HMVEC (IC50=0.13 μM), and HASMC calcium mobilization (IC50=0.17 μM), thrombin's action is blocked[1]. RWJ56110 (0.1–10 μM; 24-96 hours) has a dose-dependent inhibitory effect on endothelial cell proliferation, with a half-maximum inhibitory concentration of roughly 10 μM[2]. In a test for thymidine incorporation, RWJ56110 (0.1–10 μM; 6 hours) prevents endothelial cells from synthesizing DNA. While RWJ56110 decreases cell DNA synthesis in a dose-dependent way in endothelial cells in a fast-growing state (50–60% confluence), the inhibitory effect of PAR-1 antagonists is significantly less prominent in quiescent (100% confluent) cells[2]. Erk1/2 activation mediated by thrombin is inhibited in a concentration-dependent manner by RWJ56110 (0.1-10 μM; pretreatment for 15 min). Nevertheless, FBS (final concentration of 4%), when used to stimulate endothelial cells, partially lowers the activated levels of Erk1/2[2]. The advancement of the endothelial cell cycle is inhibited by RWJ56110 (30 μM; 24 hours). It decreases the proportion of cells in the S phase, but has less of an impact on the proportions of G1 and G2/M cells[2] |
| Cell Assay |
Western Blot Analysis[2] Cell Types: Endothelial cells Tested Concentrations: 0 μM; 3 μM; 1 μM; 3 μM; 10 μM Incubation Duration: Pretreatment for 15 min Experimental Results: Resulted in MAPK activation in Endothelial cells. Cell Cycle Analysis[2] Cell Types: Endothelial cells Tested Concentrations: 0 μM; 3 μM; 1 μM; 3 μM; 10 μM Incubation Duration: Pretreatment for 15 min Experimental Results: decreased cell number in S phase. |
| References |
[1]. Design, synthesis, and biological characterization of a peptide-mimetic antagonist for a tethered-ligand receptor. oc Natl Acad Sci U S A. 1999 Oct 26;96(22):12257-62. [2]. Blockade of angiogenesis by small molecule antagonists to protease-activated receptor-1: association with endothelial cell growth suppression and induction of apoptosis. J Pharmacol Exp Ther. 2006 Jul;318(1):246-54. |
Solubility Data
| Solubility (In Vitro) | DMSO : 200 mg/mL (231.58 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 5 mg/mL (5.79 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 5 mg/mL (5.79 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 5 mg/mL (5.79 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2089 mL | 6.0446 mL | 12.0891 mL | |
| 5 mM | 0.2418 mL | 1.2089 mL | 2.4178 mL | |
| 10 mM | 0.1209 mL | 0.6045 mL | 1.2089 mL |