Physicochemical Properties
| Molecular Formula | C28H28N6O2 |
| Molecular Weight | 480.56 |
| Exact Mass | 480.23 |
| Elemental Analysis | C, 69.98; H, 5.87; N, 17.49; O, 6.66 |
| CAS # | 2763831-53-2 |
| PubChem CID | 165437244 |
| Appearance | Off-white to light yellow solid powder |
| LogP | 4.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 36 |
| Complexity | 786 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | XYAYQAFPZOGMAA-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C28H28N6O2/c1-17(2)26(35)31-28-30-25-15-21(12-13-34(25)32-28)20-10-11-24-22(14-20)23(16-33(24)4)27(36)29-18(3)19-8-6-5-7-9-19/h5-18H,1-4H3,(H,29,36)(H,31,32,35) |
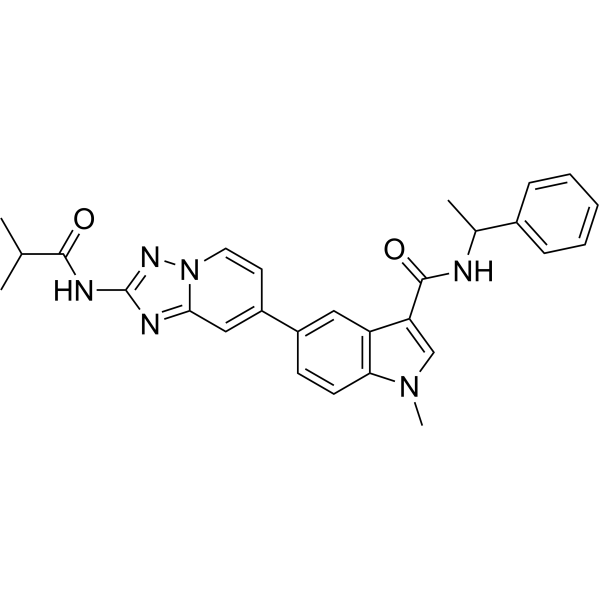
| Chemical Name | 1-methyl-5-[2-(2-methylpropanoylamino)-[1,2,4]triazolo[1,5-a]pyridin-7-yl]-N-(1-phenylethyl)indole-3-carboxamide |
| Synonyms | RI962; 2763831-53-2; SCHEMBL25830764; BDBM636322; US20230365546 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | IC50: 35.0?nM (RIPK1); EC50: 10.0 nM (HT29 cells), 4.2 nM (L929 cells), 11.4 nM (J774A.1 cells), 17.8?nM (U937 cells)[1]. |
| ln Vitro | RI-962 exhibits strong RIPK1 inhibitory activity, as evidenced by its IC50 value of 35.0 nM[1]. With EC50 values of 10.0 nM, 4.2 nM, 11.4 nM, and 17.8 nM for HT29, L929, J774A.1, and U937 cells, respectively, RI-962 has a protective effect against necroptotic death[1]. RI-962 (0-100 μM; 24 h) prevents cells from necroptosis caused by TSZ by blocking RIPK1's kinase activity[1]. |
| ln Vivo | RI-962 (ip; 40 mg/kg; once daily for 10 days) decreases inflammation in acute DSS-induced colitis and improves TNFα-induced SIRS[1]. Rats' pharmacokinetic parameters (iv, ip, po; 5, 20 mg/kg) for RI-962 [1]. AUC0–t(ng*h/mL) 4526.1 ± 546.0 1594.9 ± 891.8 6459.7 ± 1131.6 AUC0–∞ (ng* h/mL) 4538.1 ± 546.3 1604.5 ± 896.1 6609.3 ± 1121.4 Vss (L/kg) 0.4 ± 0.1 - - MRT0–∞ (h) 0.4 ± 0.0 1.8 ± 0.2 2.8 ± 0.1 CL (mL/min/kg) 18.5 ± 2.1 - - F (%) - 8.8 ± 5.0 35.7 ± 6.3 |
| Enzyme Assay |
In vitro kinase activity assays[1] In vitro kinase activity assays were conducted through the Kinase Profiling Services provided by Eurofins. The protocol for the RIPK1 assay is briefly described as follows (Protocols for other kinases are very similar and can be found in http://www.eurofins.com/pharmadiscovery). RIPK1 kinase was incubated with the test compound in assay buffer containing 8 mM MOPS (pH 7.0), 0.2 mM EDTA, 250 μM KKKSPGEYVNIEFG, 10 mM magnesium acetate, and 10 μM [γ-33P]-ATP for 15 min at room temperature. The reaction was initiated by the addition of the Mg/ATP mixture. After incubation for 40 min at room temperature, and the reaction was stopped by the addition of 3% phosphoric acid. A 10 μL portion of the reaction mixture was then spotted onto a P30 filter mat and washed four times for 4 min in 0.425% phosphoric acid and once in methanol prior to drying and scintillation counting. |
| Cell Assay |
Cell Viability Assay[1] Cell Types: HT29, L929, J774A.1, and U937 cells Tested Concentrations: 0-100 μM Incubation Duration: 24 h Experimental Results: Exerted a dose-dependent protective effect against necroptotic death. Western Blot Analysis[1] Cell Types: HT29 cells Tested Concentrations: 0-400 nM Incubation Duration: Experimental Results: Markedly inhibited the phosphorylation of RIPK1 and its downstream signaling proteins RIPK3 and MLKL in a dose- dependent manner. |
| Animal Protocol |
Animal/Disease Models: C57BL/6 female mice[1] Doses: 40 mg/kg Route of Administration: intraperitoneal (ip)for 15 min; one time/day for 10 day Experimental Results: Ameliorated TNFα-induced SIRS by inhibiting RIPK1 activity. Suppressed the RIPK1 signaling in the mouse model of DSS-induced colitis. Animal/Disease Models: SD (Sprague-Dawley) rats[1] Doses: 5, 20 mg/kg Route of Administration: intravenous (iv)(iv) (5 mg/kg), intraperitoneal (ip)(20 mg/kg) and oral (po) (20 mg/kg) Experimental Results: Had good metabolic stability in rats. |
| References |
[1]. Generative deep learning enables the discovery of a potent and selective RIPK1 inhibitor. Nat Commun. 2022 Nov 12;13(1):6891. |
| Additional Infomation |
The DSS-induced IBD experiment[1] DSS (3% w/v) was administered in drinking water ad libitum for 7 d (from day 0 to day 7). DSS solution was replaced three times on day 2, day 4, and day 6. C57BL/6 female mice were injected intraperitoneally with vehicle, RI-962 (40 mg/kg), or GSK3145095 (40 mg/kg) for 10 d (from day 0 to day 9). Three mice in each group were killed at random on day 7, and distal colon tissues were collected for analysis. The mice weight and survival rate were recorded daily. Assessment of pharmacokinetic (PK) properties[1] The PK properties of compounds were examined in male Sprague-Dawley rats (n = 3 per group, weight: 180–220 g). Compounds were dissolved in saline with 5% (v/v) DMSO plus 40% (v/v) PEG400. The animals were administered with a single dose of 5 mg/kg (intravenous injection (i.v.)), 20 mg/kg (intraperitoneal injection (i.p.) or oral gavage (p.o.)). Blood samples were collected at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 10 and 24 h, and centrifuged to isolate plasma. Subsequently, the plasma compound concentrations were determined by LC-MS/MS-13, and the PK parameters were calculated using Phoenix WinNonlin 7.0. The retrieval of hit/lead compounds with novel scaffolds during early drug development is an important but challenging task. Various generative models have been proposed to create drug-like molecules. However, the capacity of these generative models to design wet-lab-validated and target-specific molecules with novel scaffolds has hardly been verified. We herein propose a generative deep learning (GDL) model, a distribution-learning conditional recurrent neural network (cRNN), to generate tailor-made virtual compound libraries for given biological targets. The GDL model is then applied to RIPK1. Virtual screening against the generated tailor-made compound library and subsequent bioactivity evaluation lead to the discovery of a potent and selective RIPK1 inhibitor with a previously unreported scaffold, RI-962. This compound displays potent in vitro activity in protecting cells from necroptosis, and good in vivo efficacy in two inflammatory models. Collectively, the findings prove the capacity of our GDL model in generating hit/lead compounds with unreported scaffolds, highlighting a great potential of deep learning in drug discovery.[1] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0809 mL | 10.4045 mL | 20.8091 mL | |
| 5 mM | 0.4162 mL | 2.0809 mL | 4.1618 mL | |
| 10 mM | 0.2081 mL | 1.0405 mL | 2.0809 mL |