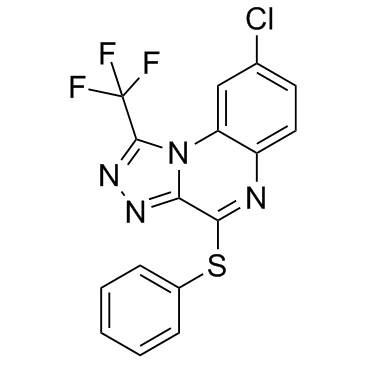
R-7050 (R7050) is a novel, potent and selective tumor necrosis factor/ TNF-α receptor antagonist acting by blocking receptor-adaptor molecule complex formation and subsequent receptor internalization, but not TNF-α ligand-receptor binding. Showing a high degree of selectivity for TNF.
Physicochemical Properties
| Molecular Formula | C16H8CLF3N4S |
| Molecular Weight | 380.774730682373 |
| Exact Mass | 380.011 |
| Elemental Analysis | C, 50.47; H, 2.12; Cl, 9.31; F, 14.97; N, 14.71; S, 8.42 |
| CAS # | 303997-35-5 |
| Related CAS # | 303997-35-5 |
| PubChem CID | 1486608 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.6±0.1 g/cm3 |
| Index of Refraction | 1.694 |
| LogP | 5.45 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 25 |
| Complexity | 477 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | FC(F)(C1=NN=C2N1C3=CC(Cl)=CC=C3N=C2SC4=CC=CC=C4)F |
| InChi Key | SUUMKHOVGVYGOP-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C16H8ClF3N4S/c17-9-6-7-11-12(8-9)24-13(22-23-15(24)16(18,19)20)14(21-11)25-10-4-2-1-3-5-10/h1-8H |
| Chemical Name | 8-chloro-4-phenylsulfanyl-1-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]quinoxaline |
| Synonyms | TNF-α Antagonist III; R-7050; 8-chloro-4-phenylsulfanyl-1-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]quinoxaline; 8-Chloro-4-(phenylthio)-1-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]quinoxaline; 8-chloro-1-(trifluoromethyl)[1,2,4]triazolo[4,3-a]quinoxalin-4-yl phenyl sulfide; 8-chloro-4-(phenylsulfanyl)-1-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]quinoxaline; R 7050; SUUMKHOVGVYGOP-UHFFFAOYSA-N; R7050; R 7050 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | TNF-α (EC50 = 0.63 μM); IL-1β (EC50 = 1.45 μM) |
| ln Vitro | R-7050 has a distinct preference for TNFα and to a slightly lesser extent, for the LPA receptor, which is a type of GPCR, as opposed to IL-1β, tyrosine kinase, or Fas receptor systems[2]. |
| ln Vivo | R-7050 reduces the neurovascular damage caused by intracerebral hemorrhage (ICH) in mice. R-7050 administration up to two hours after the injury markedly decreased blood-brain barrier opening and slowed the onset of edema 24 hours after the injury. Over the first three days following the injury, neurological results also improved. R-7050, on the other hand, had no effect on hematoma volume reduction[1]. |
| Enzyme Assay |
High-Throughput Screening [2] Compounds were dissolved in DMSO at a final stock concentration of 10 mM and stored at −80°C. Compound library screening was performed using a fully integrated, programmable robotic liquid handling system with an integrated plate reader and environmentally controlled plate carousel set at 37°C and 5% CO2. A549 cells (1 × 104/well) were seeded overnight into 96-well white flat clear-bottomed plates in 100 μl of culture medium. The next day, 100 μl aliquots from the ∼300,000-compound library prepared in the same culture medium were added at a final concentration of 10 μM in 0.2% (v/v) DMSO. Cells were preincubated with compounds for 1 hr prior to a 4 hr stimulation with either TNFα or IL-1β (1 ng/ml final concentration). At the end of the stimulation period, cells were stained for ICAM-1 using mouse anti-human CD54 monoclonal antibodyand HRP-conjugated anti-mouse IgG2a added together at final dilutions of 1:1,000 and 1:10,000, respectively. Following 1 hr of incubation at 37°C, plates were washed four times using 300 μl PBS and developed using supersignal ELISA pico chemiluminescent substrate. For EC50 determination, cells were stimulated with either TNFα (25 ng/ml) or IL-1β (10 ng/ml) only for 4 hr to avoid secondary stimulation by other cytokines produced by the cells. Image-Based NFκB Nuclear Translocation Assay [2] One day before the assay, HeLa cells were seeded into 96-well plates at a density of 2000 cells/well. On the day of assay, cells were preincubated with serially diluted test compounds (e.g. R-7050) for 1 hr and stimulated with either TNFα (25 ng/ml) or IL-1β (10 ng/ml) for 30 min at 37°C. Cells were washed twice with PBS and fixed using methanol for 1 hr at room temperature. After blocking the cells with 1% BSA in PBS-Tween 20 buffer for 1 hr, cells were treated overnight with the same buffer containing anti-NFκB p65 rabbit polyclonal antibody (1:1000 dilution) followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG staining (1:1000 dilution) for 3 hr at room temperature using a protocol described previously. Cells were also costained with 4′,6-diamidino-2-phenylindole (DAPI) for 1 hr to track the nuclear morphology. Digital images were captured using a 10× objective on a Zeiss Axiovert S100 microscope equipped with a UV filter set and a Photometrics camera. |
| Cell Assay |
Cell Death and Viability Assays [2] Cell viability was assessed using the celltiter-glo luminescent cell viability assay kit. TNFα-sensitive ME180 cells were preincubated with test compounds such as R-7050 for 1 hr at 37°C and stimulated with various concentrations of TNFα for 24 hr. At the end of the incubation period, culture media were replaced with 50 μl of prediluted celltiter-glo reagent (1:1 with PBS) and relative luminescence intensity was measured using a microtiter plate reader. Two known NFκB-pathway inhibitors (IKK2-VI and MG132) and an RNA-synthesis inhibitor (Act-D) known to enhance TNFα-induced cytotoxicity were used as controls. Caspase Assay [2] The effect of compounds on caspase activity was determined using the Caspase-Glo 3/7 assay kit. ME180 cells were preincubated with test compounds such as R-7050 for 1 hr at 37°C and stimulated with various concentrations of TNFα for 24 hr. At the end of the incubation period, culture media were replaced with 50 μl of diluted caspase-3/7 reagent (1:1 with PBS) and relative luminescence intensity was determined following the manufacturer's instructions. A proteasome inhibitor, MG132 (known to increase TNFα-induced cytotoxicity), and a general caspase inhibitor, Z-VAD-fmk, were used as controls. Immunofluorescence Analysis of TNFα Receptor Internalization [2] This study was carried out as reported by Schutze et al. Briefly, HeLa cells were seeded onto 12-well tissue culture plates containing sterile circular microscope coverslips. Following preincubation of cells with a test compound such as R-7050 or a positive control (MDC) for 1 hr at 37°C, the temperature was shifted to 4°C and biotinylated human TNFα (100 ng/ml) provided in the Fluorokine kit (NFTA0) obtained from R&D Systems was added to the culture medium. After 2 hr, cells were washed with PBS to remove unbound TNFα, and biotinylated TNFα-TNFαR complex was allowed to internalize for 10, 30, and 60 min. Cells were then washed twice with PBS and fixed using cold methanol (90%) for 30 min. Cells were again washed with PBS and blocked with Super Block solution containing 0.5% Triton X-100 for 2 hr at room temperature. Cells were stained with FITC-conjugated avidin (1:50 dilution) overnight in the same blocking solution at 4°C. Coverslips were flipped onto glass slides preloaded with a drop of Vecta-shield mounting media. Localization of the ligand-receptor complex was examined under an Axiovert S100 fluorescence microscope. |
| Animal Protocol |
Mice: A mouse collagenase model of ICH is utilized. In a nutshell, 8–10 week old male CD-1 mice are positioned in a stereotactic frame, and a burr hole with a diameter of 0.5 mm is drilled over the parietal cortex, 2.2 mm lateral to the bregma. The left striatum is injected directly from the surface of the skull 3 mm down using a 26-gauge Hamilton syringe filled with 0.04 U of bacterial type IV collagenase in 0.5 L saline. To avoid solution reflux and excessive diffusion, the syringe is depressed at 450 nL/min and left in place for a few minutes after the procedure. Sham animals undergo the same surgical procedure, except that saline is stereotactically injected rather than collagenase. After the syringe is removed, bone wax is used to close the burr hole, the incision is surgically stapled, and mice are kept warm until recovery of the righting reflex. R-7050 (6-18 mg/kg) is administered via intraperitoneal route at the time of injury or up to 2 h post-ICH. Briefly, male CD-1 mice (8–10 weeks old) were placed into a stereotactic frame and a 0.5 mm diameter burr hole was drilled over the parietal cortex, 2.2 mm lateral to the bregma. A 26-gauge Hamilton syringe, loaded with 0.04U of bacterial type IV collagenase in 0.5 μL saline, was lowered 3 mm deep from the skull surface directly into the left striatum. The syringe was depressed at a rate of 450 nL/min and left in place for several minutes after the procedure to prevent solution reflux and excess diffusion. Sham animals underwent the same surgical procedure, except that saline was stereotactically injected rather than collagenase. After the syringe was removed, bone wax was used to close the burr hole, the incision was surgically stapled, and mice were kept warm until recovery of the righting reflex. R-7050 (6–18 mg/kg) was administered via intraperitoneal route at the time of injury or up to 2h post-ICH.[1] |
| References |
[1]. Neurosci Lett. 2013 May 10; 542: 92–96. [2]. Chem Biol . 2007 Oct;14(10):1105-18. |
| Additional Infomation |
Intracerebral hemorrhage (ICH), the most common form of hemorrhagic stroke, exhibits the highest acute mortality and the worst long-term prognosis of all stroke subtypes. Unfortunately, treatment options for ICH are lacking due in part to a lack of feasible therapeutic targets. Inflammatory activation is associated with neurological deficits in pre-clinical ICH models and with patient deterioration after clinical ICH. In the present study, we tested the hypothesis that R-7050, a novel cell permeable triazoloquinoxaline inhibitor of the tumor necrosis factor receptor (TNFR) complex, attenuates neurovascular injury after ICH in mice. Up to 2h post-injury administration of R-7050 significantly reduced blood-brain barrier opening and attenuated edema development at 24h post-ICH. Neurological outcomes were also improved over the first three days after injury. In contrast, R-7050 did not reduce hematoma volume, suggesting the beneficial effects of TNFR inhibition were downstream of clot formation/resolution. These data suggest a potential clinical utility for TNFR antagonists as an adjunct therapy to reduce neurological injury and improve patient outcomes after ICH. [1] Small-molecule library screening to find compounds that inhibit TNFalpha-induced, but not interleukin 1beta (IL-1beta)-induced, intercellular adhesion molecule 1 (ICAM-1) expression in lung epithelial cells identified a class of triazoloquinoxalines. These compounds not only inhibited the TNFalpha-induced nuclear factor kappaB (NFkappaB) survival pathway but also blocked death-pathway activation. Such dual activity makes them unique against other known NFkappaB-pathway inhibitors that inhibit only a subset of TNFalpha signals leading to increased TNFalpha-induced cytotoxicity. Interestingly, these compounds inhibited association of TNFalpha receptor (TNFalphaR) I with TNFalphaR-associated death domain protein (TRADD) and receptor interacting protein 1 (RIP1), the initial intracellular signaling event following TNFalpha stimulation. Further study showed that they blocked ligand-dependent internalization of the TNFalpha-TNFalphaR complex, thereby inhibiting most of the TNFalpha-induced cellular responses. Thus, compounds with a triazoloquinoxaline scaffold could be a valuable tool to investigate small molecule-based anti-TNFalpha therapies.[2] |
Solubility Data
| Solubility (In Vitro) | DMSO: 9~25 mg/mL (23.6~65.7 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (6.57 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.57 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6263 mL | 13.1313 mL | 26.2626 mL | |
| 5 mM | 0.5253 mL | 2.6263 mL | 5.2525 mL | |
| 10 mM | 0.2626 mL | 1.3131 mL | 2.6263 mL |