Physicochemical Properties
| Molecular Formula | C6H6O3 |
| Molecular Weight | 126.111 |
| Exact Mass | 126.031 |
| CAS # | 87-66-1 |
| Related CAS # | 30813-84-4 |
| PubChem CID | 1057 |
| Appearance | White to off-white solid powder |
| Density | 1.453 |
| Boiling Point | 309 ºC |
| Melting Point | 131-135 ºC |
| Flash Point | 164.3±16.9 °C |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.677 |
| LogP | 0.29 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 9 |
| Complexity | 84.3 |
| Defined Atom Stereocenter Count | 0 |
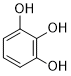
| SMILES | OC1C(O)=C(O)C=CC=1 |
| InChi Key | WQGWDDDVZFFDIG-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H |
| Chemical Name | benzene-1,2,3-triol |
| Synonyms | 2,3-Dihydroxyphenol Benzene-1,2,3-triolPyrogallol C.I. 76515 NSC 5035Fouramine Brown AP |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Pyrogallol (PG) is a reducing agent that is frequently employed as a photographic developer and in the hair dye business because it may produce free radicals, particularly superoxide anions (O2•-). Pyrogallol inhibits the development of Calu-6 and A549 lung cancer cells via depleting glutathione (GSH) and inducing apoptosis. Pyrogallol (PG) impacts mitogen-activated protein kinase (MAPK) and causes lung cancer cells to overproduce O2•-, which in turn causes apoptosis [1]. Investigations were conducted on the impact of pyrogallol on necrotic cell death and the survival of human lung fibroblasts (HPF). In these investigations, the level of inhibition or death of cell viability with or without a specific MAPK inhibitor was determined using 0, 50, or 100 µM pyrogallol. After 24 hours, treatment with 50 and 100 µM pyrogallol decreased HPF activity by roughly 40% and 65%, respectively. Treatment with a MEK inhibitor marginally increased, and treatment with a p38 inhibitor somewhat decreased, the suppression of cell viability in HPF cells treated with 50 µM pyrogallol. All MAPK inhibitors to some extent improved the inhibition of vitality in HPF cells treated with 100 µM pyrogallol; treatment with p38 inhibitor alone increased the viability of HPF control cells. Lactate dehydrogenase (LDH) release from cells was used to measure necrotic cell death. While LDH release from HPF cells was not affected by treatment with 50 µM pyrogallol, it was dramatically increased by treatment with 100 µM pyrogallol [1]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion The substance can be absorbed into the body by ingestion. Readily absorbed via skin. ...Readily absorbed from gastroenteric tract & from parenteral sites of injection. Little is absorbed through intact skin. ...readily conjugated with hexuronic, sulfuric, or other acids & excreted within 24 hr via kidneys. A fraction is excreted unchanged. Metabolism / Metabolites ...Pyrogallol /is a metabolite of tannic acid... With pyrogallol derivatives...the middle phenolic group is methylated, with catechol derivatives methylation may be meta or para, dependent on the other substituents present. Pyrogallol /is methylated by catechol o-methyl transferase to form/ 2-methyl pyrogallol. Pyrogallol in rats yields 3-methoxycatechol & 2-methoxyresorcinol. In grass yields 2-methoxyresorcinol. /From table/ Pyrogallol in beef yields purpurogallin. In tea yields purpurogallin. /From table/ For more Metabolism/Metabolites (Complete) data for Pyrogallic acid (7 total), please visit the HSDB record page. |
| Toxicity/Toxicokinetics |
Interactions ... The involvement of various molecular events in pyrogallol-mediated hepatotoxicity was deciphered by differential mRNA transcription profiles of control and pyrogallol treated mice liver. The modulatory effects of silymarin on pyrogallol-induced differentially expressed transcripts were also looked into. Swiss albino mice were treated with or without pyrogallol. In some sets of experiments, mice were also treated with silymarin 2 hr prior to pyrogallol. Total RNA was isolated from liver and polyadenylated RNA was reverse-transcribed into Cye 3 or Cye 5 labeled cDNA. Equal amounts of labeled cDNA from two different groups were mixed and hybridized with mouse 15k array. The hybridized arrays were scanned, analyzed and the expression level of each transcript was calculated. The differential expression was validated by quantitative real time polymerase chain reaction. Comparative transcription pattern showed an alteration in the expression of 183 transcripts (150 up-regulated and 33 down-regulated) associated with oxidative stress, cell cycle, cytoskeletal network, cell-cell adhesion, extra-cellular matrix, inflammation, apoptosis, cell-signaling and intermediary metabolism in pyrogallol-exposed liver and silymarin pre-treatment modulated the expression of many of these transcripts. Results obtained thus suggest that pyrogallol induces multiple molecular events leading to hepatotoxicity and silymarin effectively counteracts pyrogallol-mediated alterations. ... /This/ study was undertaken to assess the effect of resveratrol against pyrogallol-induced changes in hepatic damage markers, xenobiotic metabolizing enzymes and oxidative stress. Swiss albino mice were treated intraperitoneally, daily with pyrogallol (40 mg/kg), for one to four weeks, along with respective controls. In some set of experiments, animals were pre-treated with resveratrol (10 mg/kg), 2 hr prior to pyrogallol treatment, along with respective controls. Alanine aminotransaminase, aspartate aminotransaminase and bilirubin were measured in blood plasma and mRNA expression of cytochrome P-450 (CYP) 1A1, CYP1A2, CYP2E1, glutathione-S-transferase (GST)-ya and GST-yc, catalytic activity of CYP1A1, CYP1A2, CYP2E1, GST, glutathione reductase and glutathione peroxidase, lipid peroxidation and reduced glutathione (GSH) level were measured in liver. Resveratrol reduced pyrogallol-mediated increase in alanine aminotransaminase, aspartate aminotransaminase, bilirubin, lipid peroxidation and mRNA expression and catalytic activity of CYP2E1 and CYP1A2. Pyrogallol-mediated decrease in GST-ya and GST-yc expressions, GST, glutathione peroxidase and glutathione reductase activities and GSH content was significantly attenuated in resveratrol co-treated animals. CYP1A1 expression and catalytic activity were not altered significantly in any treated groups. The results demonstrate that resveratrol modulates pyrogallol-induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. The effect of a free radical generator pyrogallol on gastric emptying was studied in rats. Pyrogallol at doses of 25, 50, 100 and 150 mg/kg (ip) produced dose-dependent inhibition of gastric emptying. Pretreatment with vitamin C (100 and 500 mg/kg, p.o.), and vitamin E (100 and 500 mg/kg, po) significantly reversed the inhibition in gastric emptying caused by pyrogallol 100 mg/kg. However, the combination of vitamin C and vitamin E (100 mg/kg) produced synergistic effect. Glutathione (100 mg/kg iv) 5-min pretreatment also reversed the inhibition of gastric emptying caused by pyrogallol 100 mg/kg. Ondansetron (3 mg/kg, po) significantly reversed the pyrogallol effect. The effect of pyrogallol on malondialdehyde (MDA) levels and 5-HT levels in the stomach tissue was also studied. Pyrogallol at a dose of 100 mg/kg, i.p., significantly increased MDA levels and 5-HT levels in the stomach. Pretreatment with a combination of vitamin C and vitamin E (100 mg/kg, p.o.) and glutathione (100 mg/kg, i.v.) significantly ameliorated the rise in stomach tissue MDA caused by pyrogallol but had no significant effect on the rise in 5-HT levels caused by pyrogallol. The effect of different doses of 5-HT on gastric emptying was also studied. 5-HT had a differential effect on gastric emptying. The low and high doses (0.1, 0.3 and 30 mg/kg, ip) significantly inhibited the gastric emptying while doses ranging from 1 to 10 mg/kg, i.p., had no significant effect on the gastric emptying. The pretreatment with antioxidants, combination of vitamin C and vitamin E (100 mg/kg each, p.o.) and glutathione (100 mg/kg, i. v.) had no effect on the 5-HT (0.3 mg/kg, ip)-induced delay in gastric emptying. The result indicate the role of free radicals in gastric emptying, and antioxidants may be of potential therapeutic value in disease conditions where free radicals are known to be released and the gastrointestinal effects are observed as symptoms or side effects of drug therapy. This study was designed (i) to test the hypothesis that the endothelium-derived hyperpolarizing factor (EDHF) component of ACh-induced vasorelaxation and hyperpolarization of smooth muscle cells (SMCs) are impaired following exposure to superoxide anion, and (ii) to further investigate whether luteolin and apigenin induce vasoprotection at the vasoactive concentrations in rat mesenteric artery. Rat mesenteric arterial rings were isolated for isometric force recording and electrophysiological studies. Perfusion pressure of mesenteric arterial bed was measured and visualization of superoxide production was detected with fluorescent dye. 300 microM pyrogallol significantly decreased the relaxation and hyperpolarization to ACh. Luteolin and apigenin both induced vasoprotection against loss of the EDHF component of ACh-induced relaxation and attenuated the impairment of hyperpolarization to ACh. Oxidative fluorescent microtopography showed that either luteolin or apigenin significantly reduced the superoxide levels. The results suggest that superoxide anion impairs ACh-induced relaxation and hyperpolarization of SMC in resistance arteries through the impairment of EDHF mediated responses. Luteolin and apigenin protect resistance arteries from injury, implying that they may be effective in therapy for vascular diseases associated with oxidative stress. For more Interactions (Complete) data for Pyrogallic acid (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse oral 300 mg/kg LD50 Mouse ip 400 mg/kg LD50 Mouse sc 566 mg/kg LD50 Rabbit oral 1600 mg/kg |
| References |
[1]. MAPK inhibitors enhance cell death in pyrogallol-treated human pulmonary fibroblast cells via increasing O2•- levels. Oncol Lett. 2017 Jul;14(1):1179-1185. |
| Additional Infomation |
Therapeutic Uses /Experimental Therapy/ ... Pyrogallol had highly cytotoxic effect on human lung cancer cell lines and less effect on human bronchial epithelium cell line. This study was performed to investigate the beneficial effect of pyrogallol on human lung cancer cell lines - H441 (lung adenocarcinoma) and H520 (lung squamous cell carcinoma). The MTT (cytotoxic) data showed the inhibition growth of lung cancer cells followed pyrogallol treatment. The cell cycle of lung cancer cells was arrested in G2/M phase using flow cytometry. Using Western blot analysis, the cell cycle related proteins - cyclin B1 and Cdc25c were decreased in a time-dependent manner and the phosphorylated Cdc2 (Thr14) was increased within 4h pyrogallol treatment. Moreover, the higher cleavage of poly (ADP)-ribose polymerase (PARP), the increased of Bax concurrent with the decreased of Bcl-2 indicated that pyrogallol treatment resulted in apoptosis of lung cancer cells. The cell apoptosis was also directly demonstrated using Annexin V-FITC and TUNEL stain. Additionally, the tumoricidal effect of pyrogallol was measured using a xenograft nude mice model. After 5 weeks of pyrogallol treatment could cause the regression of tumor. Taking in vitro and in vivo studies together, these results suggest that pyrogallol can be developed as a promising anti-lung cancer drug particular for the non-small cell lung cancer (NSCLC). |
Solubility Data
| Solubility (In Vitro) |
DMSO : ≥ 100 mg/mL (~792.96 mM) H2O : ~50 mg/mL (~396.48 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (19.82 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (19.82 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (19.82 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 130 mg/mL (1030.85 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.9296 mL | 39.6479 mL | 79.2959 mL | |
| 5 mM | 1.5859 mL | 7.9296 mL | 15.8592 mL | |
| 10 mM | 0.7930 mL | 3.9648 mL | 7.9296 mL |