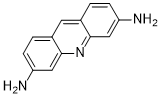
Proflavine, an acridine-derived fluorescent dye, is a slow-acting disinfectant with bacteriostatic action against many Gram-positive bacteria but less effective against Gram-negative organisms. Proflavine can be used as a rapid stain for cytologic examination of biological specimens. Proflavine fluorescently stains cell nuclei and cytoplasmic structures, owing to its small amphipathic structure and ability to intercalate DNA. In this manuscript, we demonstrated the use of proflavine as a rapid cytologic dye on a number of specimens, including normal exfoliated oral squamous cells, cultured human oral squamous carcinoma cells, and leukocytes derived from whole blood specimens using a custom-built, portable, LED-illuminated fluorescence microscope. No incubation time was needed after suspending cells in 0.01% (w/v) proflavine diluted in saline. Images of proflavine stained oral cells had clearly visible nuclei as well as granular cytoplasm, while stained leukocytes exhibited bright nuclei, and highlighted the multilobar nature of nuclei in neutrophils. We also demonstrated the utility of quantitative analysis of digital images of proflavine stained cells, which can be used to detect significant morphological differences between different cell types. Proflavine stained oral cells have well-defined nuclei and cell membranes which allowed for quantitative analysis of nuclear to cytoplasmic ratios, as well as image texture analysis to extract quantitative image features.
Physicochemical Properties
| Molecular Formula | C13H11N3 |
| Molecular Weight | 209.25 |
| Exact Mass | 209.095 |
| Elemental Analysis | C, 74.62; H, 5.30; N, 20.08 |
| CAS # | 92-62-6 |
| Related CAS # | Proflavine hemisulfate;1811-28-5 |
| PubChem CID | 7099 |
| Appearance | Yellow needles from alcohol. Solutions are brownish and when diluted are fluorescent. |
| Density | 1.346 g/cm3 |
| Boiling Point | 506.9ºC at 760 mmHg |
| Melting Point | 260ºC |
| Flash Point | 292.9ºC |
| Index of Refraction | 1.833 |
| LogP | 3.714 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 16 |
| Complexity | 232 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | N1C2C(=CC=C(C=2)N)C=C2C=1C=C(C=C2)N |
| InChi Key | WDVSHHCDHLJJJR-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C13H11N3/c14-10-3-1-8-5-9-2-4-11(15)7-13(9)16-12(8)6-10/h1-7H,14-15H2 |
| Chemical Name | Acridine, 3,6-diamino- |
| Synonyms | acridine-3,6-diamine; 3,6-ACRIDINEDIAMINE; Isoflav base; |
| HS Tariff Code | 2934.99.03.00 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | proflavine can be used to rapidly stain fresh cells for cytologic analysis. Proflavine is an effective fluorescent contrast agent for exfoliated oral squamous cells, highlighting nuclear and cytoplasmic structures, including keratohyalin granules in mature cells. Structural definition in proflavine-stained cells is comparable to Giemsa or Papanicolaou staining. As opposed to the multi-step procedure of Papanicolaou staining, proflavine staining requires only the addition of the dye to the cell medium before mounting on a slide, eschewing the need for fixation. Compared to traditional pathology stains, proflavine is cost-effective, requires little setup and materials, and does not require lengthy staining time . Additionally, proflavine can rapidly stain freshly collected leukocytes, clearly showing the multilobar structure of granulocytes in contrast to other cellular components. Finally, fluorescence images of proflavine stained cells can be analyzed quantitatively to highlight statistically significant differences in cell types based on morphologic features.[4] |
| Cell Assay |
The samples of exfoliated cells were washed in PBS three times and incubated in 1% albumin from bovine serum for 5 min. The BSA was removed and the cells were stained with a solution of 0.01% (w/v) proflavine in PBS. Additionally, to collect immature oral epithelial cells, a cotton swab was used to collect normal oral cells from a volunteer. The swab was mixed in a 0.01% (w/v) proflavine solution to suspend and stain the cells. [4] Passaged CAL 27 cells (up to passage number four) were centrifuged at 200g for 5 min and the media removed. The cells were washed once with PBS by centrifuging at 200g for 5 min, decanting the supernatant, and resuspending in 1% BSA for 5 min. The BSA was removed and the cells were stained with the 0.01% (w/v) proflavine solution.[4] The samples of whole blood were stained at a final concentration of 0.01% (w/v) proflavine solution.[4] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion The uptake of the fluorescent drug proflavine was measured in suspensions of hepatocytes from normal and carcinogen (2-acetylaminofluorine, AAF)-fed rats by flow cytometry. Drug uptake into hepatocytes from carcinogen-fed animals was consistently lower than that into hepatocytes from normal animals. Isolated nuclei, prepared from the livers of normal and AAF-fed rats showed similar proflavine uptake. Drug uptake into hepatocytes from AAF-fed animals, however, was increased by prior exposure to a metabolic inhibitor. Thus, differences in drug uptake may reflect changes in the cell membrane, together with an alteration in the metabolic integrity of the cells. The uptake of drug in hepatocytes from AAF-fed rats was uniformly low within each cell preparation. However, drug uptake varied not only between tumours arising in the livers of these animals but also within each tumour cell preparation. This study indicates that flow cytometry can provide an effective means for analysing drug uptake into cell populations arising during hepatocarcinogenesis. 1. The disposition of proflavine (PRO) and acriflavine (ACR) were examined in channel catfish after intravascular (i.v.) dosing (1 mg/kg) or waterborne exposure (10 mg/l for 4 h). 2. After i.v. dosing, plasma concentration-time profiles of parent PRO and ACR were best described by two- and three-compartment pharmacokinetic models respectively. Terminal elimination half-lives of PRO and ACR in plasma were 8.7 and 11.4 h respectively. 3. In animals dosed with 14C-PRO or 14C-ACR, total drug equivalent concentrations were highest in the excretory organs and lowest in muscle, fat and plasma. In PRO-dosed animals, residues in the liver and trunk kidney were composed primarily of glucuronosyl and acetyl conjugates of PRO; residues in muscle were composed mostly (> 95%) of the parent drug. In ACR-dosed animals, the parent compound comprised > 90% of the total residues in all tissues examined. 4. PRO and ACR were poorly absorbed in catfish during waterborne exposure. At the end of a 4-h exposure, parent PRO and ACR concentrations in muscle were 0.064 and 0.020 microgram/g respectively. Levels in muscle declined below the limit of determination (0.005 microgram/g) within 1-2 weeks. Metabolism / Metabolites Proflavine (3,6-diaminoacridine) has potential for use as an antiinfective in fish, and its metabolism by rainbow trout was therefore studied. Fourteen hours after intraarterial bolus administration of 10 mg/kg of proflavine, three metabolites were found in liver and bile, and one metabolite was found in plasma using reversed-phase HPLC with UV detection at 262 nm. Treatment with hydrochloric acid converted the three metabolites to proflavine, which suggested that the metabolites were proflavine conjugates. Treatment with beta-glucuronidase and saccharic acid 1,4-lactone, a specific beta-glucuronidase inhibitor, revealed that two metabolites were proflavine glucuronides. For determination of UV-VIS absorption and mass spectra, HPLC-purified metabolites were isolated from liver. Data from these experiments suggested that the proflavine metabolites were 3-N-glucuronosyl proflavine (PG), 3-N-glucuronosyl,6-N-acetyl proflavine (APG), and 3-N-acetylproflavine (AP). The identities of the metabolites were verified by chemical synthesis. When synthetic PG and AP were compared with the two metabolites isolated from trout, they had the same molecular weight as determined by matrix-assisted, laser desorption ionization, time-of-flight MS. In addition, they coeluted on HPLC under different mobile phase conditions. Finally, the in vitro incubation with liver subcellular preparations confirmed this characterization and provided the evidence that APG can be formed by glucuronidation of AP or acetylation of PG. A liquid chromatographic (LC) method was developed for determination of acriflavine (ACR) and proflavine (PRO) residues in channel catfish muscle. Residues were extracted with acidified methanol solution, and extracts were cleaned up with C18 solid-phase extraction columns. Residue concentrations were determined on an LC cyano column, with spectrophotometric detection at 454 nm. Catfish muscle was individually fortified with ACR (purified from commercial product) and PRO at concentrations of 5, 10, 20, 40, and 80 ppb (5 replicates per level). Mean recoveries from fortified muscle at each level ranged from 86 to 95%, with relative standard deviations (RSDs) of 2.5 to 5.7%. The method was applied to incurred residues of ACR and PRO in muscle after waterborne exposure of channel catfish to commercial acriflavine (10 ppm total dye for 4 h). RSDs for incurred residues of ACR and PRO were in the same range as those for fortified muscle. Low residue concentrations (< 1% of exposure water concentration) suggested poor absorption of ACR and PRO in catfish. |
| Toxicity/Toxicokinetics |
Non-Human Toxicity Values LD50 mouse intraperitoneal 50 mg/kg LD50 mouse subcutaneous 140 mg/kg |
| References |
[1]. Proflavine an acridine DNA intercalating agent and strong antimicrobial possessing potential properties of carcinogen. Karbala International Journal of Modern Science. 2017 Dec, 3(4): 272-278. [2]. Isolation of proflavine as a blocker of G protein-gated inward rectifier potassium channels by a cell growth-based screening system. Neuropharmacology. 2016 Oct;109:18-28. [3]. Determination of proflavine in rat whole blood without sample pretreatment by laser desorption postionization mass spectrometry. Anal Bioanal Chem. 2017 Apr;409(11):2813-2819. [4]. PLoS One.2015 May 11;10(5):e0125598. |
| Additional Infomation |
3,6-diaminoacridine is an aminoacridine that is acridine that is substituted by amino groups at positions 3 and 6. A slow-acting bacteriostat that is effective against many Gram-positive bacteria (but ineffective against spores), its salts were formerly used for treatment of burns and infected wounds. It has a role as an antiseptic drug, a carcinogenic agent, an antibacterial agent, a chromophore and an intercalator. It is a conjugate base of a 3,6-diaminoacridine(1+). 3,6-Diaminoacridine. Topical antiseptic used mainly in wound dressings. Proflavine Hemisulfate is the hemisulfate salt form of proflavine, an acridine-derived fluorescent contrast and disinfectant agent that can potentially be used for cellular imaging and antiseptic purposes. Upon topical application of proflavine hemisulfate, proflavine diffuses into cells and intercalates into DNA, thereby accumulating in and staining the nucleus. During fluorescence imaging, the cell nuclei can be visualized. This allows nuclear morphometry and the identification of cancer cells. In addition, proflavine exerts its antibacterial effect by binding to bacterial DNA, thereby disrupting DNA synthesis and halting bacterial cell growth. Topical antiseptic used mainly in wound dressings. Drug Indication Topical antiseptic used mainly in wound dressings. Mechanism of Action Proflavine acts by interchelating DNA (intercalation), thereby disrupting DNA synthesis and leading to high levels of mutation in the copied DNA strands. This prevents bacterial reproduction. The ability of proflavine (3,6-diaminoacridine) and its 2,7-dimethyl, 2,7-diethyl, 2,7-diisopropyl and 2,7-di-tert.-butyl derivatives to induce the 'petite' mutation in Saccharomyces cerevisiae has been studied in relation to the DNA-binding properties of the compounds. The nature of the binding has been investigated by nuclear magnetic resonance techniques, and the results support and clarify earlier suggestions that the first 3 members of the series intercalate into DNA while the diisopropyl and di-tert.-butyl compounds do not. Toxicity of the drugs was primarily associated with their mode of DNA binding, but lipophilicity had an important secondary effect. It seems likely that the toxic properties of the more lipophilic DNA-intercalating members of the series mask their potential for 'petite' mutagenesis. The toxicities of several aminoacridines were measured against pathogenic strains of both Gram-positive (Staphylococcus aureus, Enterococcus faecalis, Bacillus cereus) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa) organisms. In several cases, illumination at a light dose of 6.3 J/cm2 resulted in considerable decreases in the minimum lethal drug concentrations required, giving up to 50-fold increases in bactericidal activity. Derivatives of 9-aminoacridine (aminacrine) exhibited phototoxicity against one or more of the test organisms, but the established photosensitizing acridines proflavine and acridine orange were photobactericidal against all strains. Therapeutic Uses /Exptl/ We used the photodynamic inactivation technique with proflavine as the photoactive dye to treat herpetic epithelial keratitis in a preliminary study of patients who had idoxuridine toxicity or resistance. A comparative study with idoxuridine in treating dendritic ulcerations of the cornea showed a good therapeutic effect. But the investigation was suspended when adverse reactions, consisting of a generalized epithelial keratitis and an anterior uveitis, possibly of phototoxic origin, developed in a few patients receiving treatment. The ulcers treated by photodynamic inactivation apparently healed by a process of "debridement" followed by subsequent re-epithelialization. ... Proflavine wool is used by many surgeons in the UK as a dressing that can be moulded to conform to the contours of a corrected prominent ear. ... Drug Warnings Proflavine allergy is uncommon, occurring in approximately 6% of patients attending contact dermatitis clinics. Proflavine wool is used by many surgeons in the UK as a dressing that can be moulded to conform to the contours of a corrected prominent ear. It may have bacteriostatic properties. We present a case where contact dermatitis in response to proflavine developed after pinnaplasty. This caused diagnostic confusion, a lengthened hospital stay and an unsightly hypertrophic scar. Pharmacodynamics Proflavine is an acriflavine derivative which is a disinfectant bacteriostatic against many gram-positive bacteria. Proflavine is toxic and carcinogenic in mammals and so it is used only as a surface disinfectant or for treating superficial wounds. |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~23 mg/mL ( ~109.91 mM ) Ethanol : ~2 mg/mL |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.7790 mL | 23.8949 mL | 47.7897 mL | |
| 5 mM | 0.9558 mL | 4.7790 mL | 9.5579 mL | |
| 10 mM | 0.4779 mL | 2.3895 mL | 4.7790 mL |