Physicochemical Properties
| Molecular Formula | C29H32CL2N6 |
| Molecular Weight | 541.547554016113 |
| Exact Mass | 540.244 |
| CAS # | 1261394-71-1 |
| Related CAS # | Piperaquine;4085-31-8 |
| PubChem CID | 122262 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 721.1±60.0 °C at 760 mmHg |
| Flash Point | 389.9±32.9 °C |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.664 |
| LogP | 5.15 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 37 |
| Complexity | 655 |
| Defined Atom Stereocenter Count | 0 |
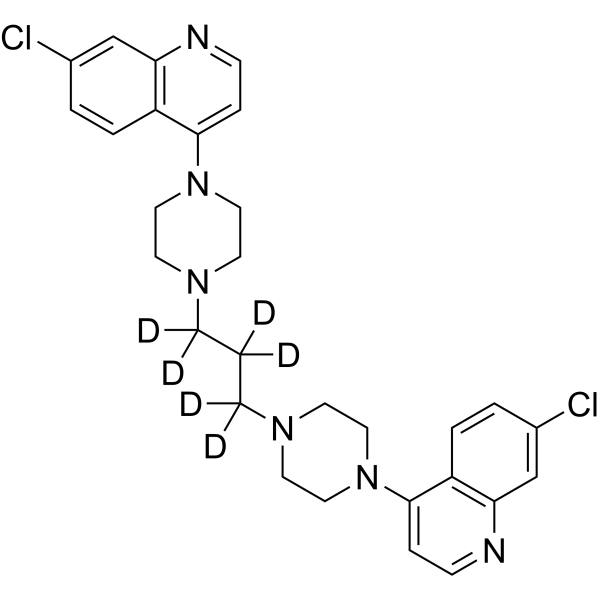
| SMILES | ClC1C=CC2C(C=1)=NC=CC=2N1CCN(CC1)C([2H])([2H])C([2H])([2H])C([2H])([2H])N1CCN(C2C=CN=C3C=C(C=CC=23)Cl)CC1 |
| InChi Key | UCRHFBCYFMIWHC-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C29H32Cl2N6/c30-22-2-4-24-26(20-22)32-8-6-28(24)36-16-12-34(13-17-36)10-1-11-35-14-18-37(19-15-35)29-7-9-33-27-21-23(31)3-5-25(27)29/h2-9,20-21H,1,10-19H2 |
| Chemical Name | 7-chloro-4-[4-[3-[4-(7-chloroquinolin-4-yl)piperazin-1-yl]propyl]piperazin-1-yl]quinoline |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Drug compounds have included stable heavy isotopes of carbon, hydrogen, and other elements, mostly as quantitative tracers while the drugs were being developed. Because deuteration may have an effect on a drug's pharmacokinetics and metabolic properties, it is a cause for concern [1]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Piperaquine is slowly absorbed and exhibits multiple peaks in its plasma concentration curve suggestive of enterohepatic recycling occurring alongside the absorption process. Due to this complication there is no discreet value for bioavailability but piperaquine is highly absorbed into systemic circulation. When taken with food, Cmax increases by 217% and mean exposure increases by 177%. Tmax is not affected by food and remains around 5 h. Piperaquine has been observed to accumulate more in females to a degree of 30-50% more than males. It also collects in red blood cells similar to [DB11638]. Piperaquine is mainly excreted in the feces with a negligible amount in the urine. Piperaquine is thought to distribute into a central compartment with an apparent volume of 26.7 L/kg, and two peripheral compartments with apparent volumes of 76.8 L/kg and 617 L/kg. These combine for a total volume of distribution of 720.5 L/kg. The mean apparent total clearance has been observed to be 1.12 L/h/kg in adult malaria patients. Metabolism / Metabolites Piperaquine undergoes N-dealkylation, separating its aliphatic bridge from one of the nitrogen-containing rings. The resulting aldehyde is then oxidized to a carboxylic acid to form metabolite 1 (M1). The same nitrogen-containing rings can also undergo hydroxylation at one of two sites to form M3 or M4. M2 is formed via N-oxidation of one of the nitrogens in the quinoline groups at either side of the molecule. M5 results when both of these nitrogens are oxidized. M1 and M2 are the major metabolism products. Each of these metabolites were observed in the urine. Biological Half-Life The terminal elimination half-life was observed to be 576h or 24 days. This is thought to be due to the extensive distribution of piperaquine. |
| Toxicity/Toxicokinetics |
Protein Binding Piperaquine's binding to plasma proteins is considered to be virtually complete. It has been measured to be >99% in humans, rats, and dogs. |
| References |
[1]. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019 Feb;53(2):211-216. [2]. Pharmacokinetics and pharmacodynamics of piperaquine in a murine malaria model. Antimicrob Agents Chemother. 2008 Jan; 52(1): 306-11. [3]. Piperaquine: a resurgent antimalarial drug. Drugs. 2005; 65(1): 75-87. |
| Additional Infomation |
Piperaquine is an aminoquinoline that is 1,3-di(piperazin-1-yl)propane in which the nitrogen at position 4 of each of the piperazine moieties is replaced by a 7-chloroquinolin-4-yl group. It has a role as an antimalarial. It is a N-arylpiperazine, an organochlorine compound and an aminoquinoline. Piperaquine is an antimalarial agent first synthesized in the 1960's and used throughout China. Its use declined in the 1980's as piperaquine resistant strains of *Plasmodium falciparum* appeared and artemisinin derivatives became available. It has come back into use in combination with the artemisinin derivative [DB11638] as part of the combination product Eurartesim. Eurartesim was first authorized for market by the European Medicines Agency in October 2011. Drug Indication For the treatment of uncomplicated *Plasmodium falciparum* infection in adults, children, and infants aged 6 months and up weighing over 5 kg. Used in combination with [DB11638]. FDA Label Mechanism of Action The mechanism of piperaquine inhibition of the haem detoxification pathway is unknown but is expected to be similar to that of [DB00608]. Pharmacodynamics Piperaquine inhibits the P. Falciparum parasite's haem detoxification pathway. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8466 mL | 9.2328 mL | 18.4655 mL | |
| 5 mM | 0.3693 mL | 1.8466 mL | 3.6931 mL | |
| 10 mM | 0.1847 mL | 0.9233 mL | 1.8466 mL |