Physicochemical Properties
| Molecular Formula | C4H11N2O2PCL2 |
| Molecular Weight | 221.02 |
| Exact Mass | 219.994 |
| CAS # | 10159-53-2 |
| Related CAS # | Phosphoramide mustard cyclohexanamine;1566-15-0 |
| PubChem CID | 96356 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.474g/cm3 |
| Boiling Point | 363.5ºC at 760mmHg |
| Flash Point | 173.6ºC |
| Vapour Pressure | 2.84E-06mmHg at 25°C |
| Index of Refraction | 1.525 |
| LogP | 1.525 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 11 |
| Complexity | 151 |
| Defined Atom Stereocenter Count | 0 |
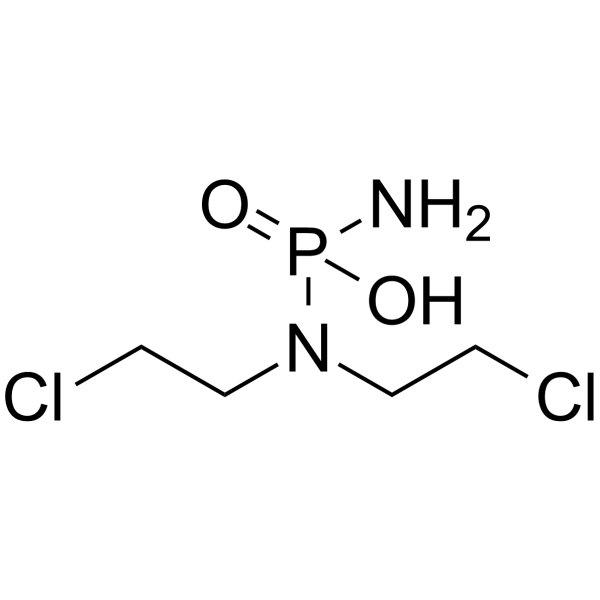
| SMILES | C(CN(CCCl)P(=O)(N)O)Cl |
| InChi Key | RJXQSIKBGKVNRT-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C4H11Cl2N2O2P/c5-1-3-8(4-2-6)11(7,9)10/h1-4H2,(H3,7,9,10) |
| Chemical Name | amino-[bis(2-chloroethyl)amino]phosphinic acid |
| Synonyms | Phosphoramide mustard |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | DNA Alkylator |
| ln Vitro | In order to prevent DNA strand separation during replication, phosphoramide mustard forms cross-linked DNA adducts, which are toxic[1]. In rat spontaneously immortalized granulosa cells (SIGCs), phosphoramide mustard (3-6 μM; 48 hours) decreases cell viability[1]. Ovarian DNA damage and DNA adduct formation are induced by mustard (3-6 μM; 24-48 hours)[1]. DNA damage responses (DDR) gene mRNA expression levels and DDR proteins are increased by phosphoramide mustard (3-6 μM; 24-48 hours)[1]. |
| ln Vivo | In rats, phosphorus mustard (2.1-20.7 mg/kg; ip; daily; for 5 days) suppresses the formation of subcutaneous tumors[2]. After intravenous treatment, phosphoramide mustard has terminal elimination half-lives (rat 15.1 min; rat 59.4 mg/kg)[2]. |
| Cell Assay |
Cell Viability Assay[1] Cell Types: SIGCs Tested Concentrations: 0.5 μM, 1 μM, 3 μM, 6 μM Incubation Duration: 48 hrs (hours) Experimental Results: decreased cell viability at concentrations of 3 μM and higher. RT-PCR[1] Cell Types: SIGCs Tested Concentrations: 3 μM, 6 μM Incubation Duration: 24 hrs (hours), 48 hrs (hours) Experimental Results: Increased DDR gene mRNA expression levels. Western Blot Analysis[1] Cell Types: SIGCs Tested Concentrations: 3 μM, 6 μM Incubation Duration: 24 hrs (hours), 48 hrs (hours) Experimental Results: Generally increased DDR proteins. |
| Animal Protocol |
Animal/Disease Models: Rat, subcutaneously (sc) implanted Walker 256 carcinosarcoma tumor[2] Doses: 2.1 mg/kg, 4.8 mg/kg, 10.4 mg /kg, 20.7 mg/kg Route of Administration: intraperitoneal (ip)injection, one time/day, for 5 days Experimental Results: Required to produce 50% inhibition of subcutaneous (sc)tumor growth with dose of 12 mg/kg. Animal/Disease Models: Rats[2] Doses: 59.4 mg /kg (pharmacokinetic/PK Analysis) Route of Administration: intravenous (iv)injection Experimental Results: T1/2 (15.1 min). |
| References |
[1]. Phosphoramide mustard exposure induces DNA adduct formation and the DNA damage repair response in rat ovarian granulosa cells. Toxicol Appl Pharmacol. 2015 Feb 1; 282(3): 252–258. [2]. Brain and plasma pharmacokinetics and anticancer activities of cyclophosphamide and phosphoramide mustard in the rat. Cancer Chemother Pharmacol. 1990;27(1):1-7. |
| Additional Infomation |
Phosphoramide mustard is a nitrogen mustard and a phosphorodiamide. Phosphoramide Mustard is one of a number of chemically-related alkylating agents with antineoplastic properties. The prototype of this group of agents is cyclophosphamide. Most phosphoramide mustards are administered as prodrugs that undergo reductive activation in hypoxic environments to yield cytotoxic metabolites. These agents alkylate and crosslink DNA, resulting in inhibition of DNA replication. Phosphoramide mustards are also immunosuppressants, mutagens and teratogens. (NCI04) |
Solubility Data
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5245 mL | 22.6224 mL | 45.2448 mL | |
| 5 mM | 0.9049 mL | 4.5245 mL | 9.0490 mL | |
| 10 mM | 0.4524 mL | 2.2622 mL | 4.5245 mL |