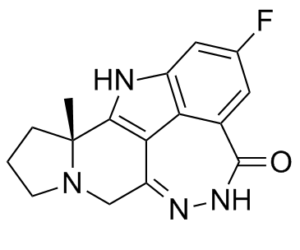
Pamiparib (also known as BGB-290; trade name in China: Baihuize) is a novel, potent and selective inhibitor of PARP1/2 approved in China for cancer treatment. It uses a mechanism known as "synthetic lethality" to destroy tumor cells. Compared to other PARP enzymes, dapagliparib exhibits a high degree of selectivity. Its DMPK (drug metabolism and pharmacokinetic) profiles are favorable. Using the base-excision repair (BER) pathway, pampiparib binds to PARP specifically and inhibits PARP from repairing single-strand DNA breaks. This process increases the accumulation of DNA strand breaks, causes genomic instability, and ultimately results in apoptosis. Pamiparib has the ability to counteract tumor cells' resistance to chemotherapy and radiation, as well as increase the cytotoxicity of agents that damage DNA.
Physicochemical Properties
| Molecular Formula | C16H15FN4O | |
| Molecular Weight | 298.31 | |
| Exact Mass | 298.122 | |
| Elemental Analysis | C, 64.42; H, 5.07; F, 6.37; N, 18.78; O, 5.36 | |
| CAS # | 1446261-44-4 | |
| Related CAS # |
|
|
| PubChem CID | 135565554 | |
| Appearance | Light yellow to yellow solid powder | |
| Density | 1.7±0.1 g/cm3 | |
| Index of Refraction | 1.829 | |
| LogP | 0.02 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 0 | |
| Heavy Atom Count | 22 | |
| Complexity | 566 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | FC1=CC2C(NN=C3C4C=2C(=C1)NC=4[C@@]1(C)CCCN1C3)=O |
|
| InChi Key | DENYZIUJOTUUNY-MRXNPFEDSA-N | |
| InChi Code | InChI=1S/C16H15FN4O/c1-16-3-2-4-21(16)7-11-13-12-9(15(22)20-19-11)5-8(17)6-10(12)18-14(13)16/h5-6,18H,2-4,7H2,1H3,(H,20,22)/t16-/m1/s1 | |
| Chemical Name | (2R)-14-fluoro-2-methyl-6,9,10,19-tetrazapentacyclo[14.2.1.02,6.08,18.012,17]nonadeca-1(18),8,12(17),13,15-pentaen-11-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PARP2 ( IC50 = 0.11 nM ); PARP1 ( IC50 = 0.83 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | BGB-290 showed excellent selectivity over other PARP enzymes and significant potency for PARP1/2 (IC50 = 0.83 and 0.11 nM, respectively) in the biochemical tests. The assay used to measure BGB-290's DNA-trapping activity was the fluorescence polarization (FP) binding method. BGB-290 exhibited a strong ability to trap DNA, with an IC50 of 13 nM. BGB-290 inhibited intracellular PAR formation in the cellular assays, with an IC50 of 0.24 nM. BGB-290 had a strong effect on tumor cell lines with homologous recombination defects. In an MDA-MB-436 (BRCA1 mutant) breast cancer xenograft, oral administration of BGB-290 led to time- and dose-dependent inhibition of PARylation, which correlated well with the tumor drug concentrations. BGB-290 produced PAR inhibition that was more persistent than olaparib. BGB-290 showed remarkable anti-tumor activity in this model, more than ten times more potent than olaparib, which is consistent with this finding. | ||
| Cell Assay | Three of the seven SCLC cell lines that were tested showed sensitivity to BGB-290. Using patient biopsy samples from Beijing Cancer Hospital, internal SCLC primary tumor models were created. Eight primary tumor models of SCLC were used to assess the anti-tumor activities of BGB-290, either alone or in conjunction with etoposide/carboplatin (E/C). In these models, BGB-290 exhibited only marginal single agent activity. In line with the clinical response seen in these patients, six of the eight models (or75%) showed sensitivity to E/C treatment. In these chemo-sensitive models, the addition of BGB-290 as maintenance therapy or concurrent treatment greatly extended the duration of the response. BGB-290 and E/C combo had less of an impact in the two chemo-insensitive models. Throughout the trial, BGB-290 was well tolerated as an addition to the chemotherapy regimen. | ||
| Animal Protocol |
|
||
| References |
[1]. Fused tetra or penta-cyclic dihydrodiazepinocarbazolones as parp inhibitors. WO 2013097225 A1. [2]. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019 Sep;20(9):1306-1315. [3]. Abstract 1653: BGB-290: A highly potent and specific PARP1/2 inhibitor potentiates anti-tumor activity of chemotherapeutics in patient biopsy derived SCLC models. Cancer Research. August 2015, Volume 75, Issue 15. [4]. Abstract 3505: Inhibition of PARP activity by BGB-290 potentiates efficacy of NSC 362856 in patient derived xenografts of glioblastoma multiforme. Cancer Research. August 2015, Volume 75, Issue 15. |
||
| Additional Infomation |
Pamiparib is under investigation in clinical trial NCT03933761 (Pamiparib in Fusion Positive, Reversion Negative High Grade Serous Ovarian Cancer or Carcinosarcoma With BRCA1/2 Gene Mutations If Progression on Substrate Poly ADP Ribose Polymerase Inhibitbor (PARPI) or Chemotherapy). Pamiparib is an orally bioavailable inhibitor of the nuclear enzyme poly(ADP-ribose) polymerase (PARP), with potential antineoplastic activity. Upon administration, pamiparib selectively binds to PARP and prevents PARP-mediated repair of single-strand DNA breaks via the base-excision repair (BER) pathway. This enhances the accumulation of DNA strand breaks, promotes genomic instability, and eventually leads to apoptosis. PARP is activated by single-strand DNA breaks and, subsequently, catalyzes post-translational ADP-ribosylation of nuclear proteins which then transduce signals to recruit other proteins to repair damaged DNA. Pamiparib may both potentiate the cytotoxicity of DNA-damaging agents and reverse tumor cell chemo- and radioresistance. Drug Indication Treatment of gastric and gastroesophageal junction adenocarcinoma |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.25 mg/mL (7.54 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 5%DMSO+ 40%PEG300+ 5%Tween 80+ 50%ddH2O: 3.0mg/ml (10.06mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3522 mL | 16.7611 mL | 33.5222 mL | |
| 5 mM | 0.6704 mL | 3.3522 mL | 6.7044 mL | |
| 10 mM | 0.3352 mL | 1.6761 mL | 3.3522 mL |