Physicochemical Properties
| Molecular Formula | C29H29CLN6O5S |
| Molecular Weight | 609.10 |
| Exact Mass | 608.16 |
| CAS # | 2785323-62-6 |
| PubChem CID | 162641046 |
| Appearance | White to yellow solid powder |
| Density | 1.453±0.06 g/cm3(Temp: 20 °C; Press: 760 Torr)(Predicted) |
| LogP | 4.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 42 |
| Complexity | 1030 |
| Defined Atom Stereocenter Count | 0 |
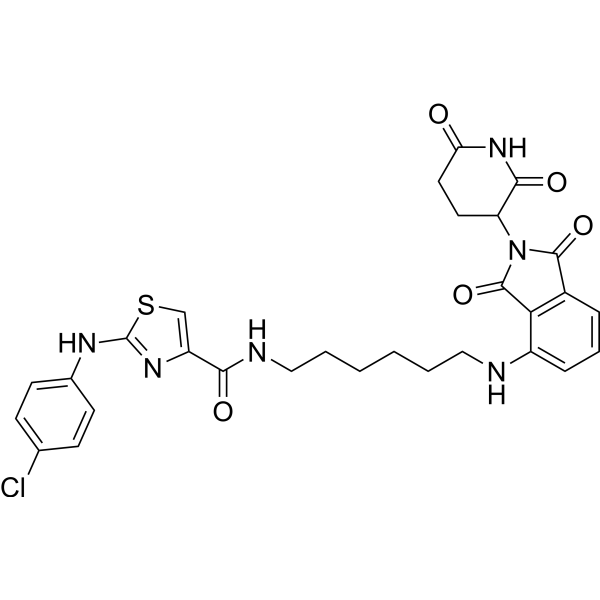
| SMILES | ClC1C=CC(=CC=1)NC1=NC(=CS1)C(NCCCCCCNC1=CC=CC2C(N(C(C=21)=O)C1C(NC(CC1)=O)=O)=O)=O |
| InChi Key | UWYLVQJEOCYCEW-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C29H29ClN6O5S/c30-17-8-10-18(11-9-17)33-29-34-21(16-42-29)25(38)32-15-4-2-1-3-14-31-20-7-5-6-19-24(20)28(41)36(27(19)40)22-12-13-23(37)35-26(22)39/h5-11,16,22,31H,1-4,12-15H2,(H,32,38)(H,33,34)(H,35,37,39) |
| Chemical Name | 2-(4-chloroanilino)-N-[6-[[2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl]amino]hexyl]-1,3-thiazole-4-carboxamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Cereblon |
| ln Vitro | PROTAC-O4I2 preferentially degrades SF3B1 and prevents the formation of tumors in cells by introducing thalidomide into the ubiquitin E3 ligase cereblon (CRBN) [1]. STAT-O4I2 inhibits and degrades SF3B1 in K562 cells. SF3B1 WT, SF3B1 OE, and SF3B1 K700E cells are non-proliferatively affected by PROTAC-O4I2, with IC50 values of 228, 63, and 90 nM, respectively [1]. With an IC50 value of 0.244 μM in K562 cells, PROTAC-O4I2 causes FLAG-SF3B1 degradation in a concentration-dependent manner [1]. |
| ln Vivo | PROTAC-O4I2 (10 μM) inhibits the maintenance and development of the tumor, hence considerably increasing the survival rate of Drosophila in the intestinal tumor model [1]. |
| Cell Assay |
Cell Proliferation Assay[1] Cell Types: K562 control cells (WT), cells overexpressing SF3B1 (OE), and cells expressing SF3B1K700E (K700E) Tested Concentrations: 1 pM, 0.1 nM, 10 nM, 1 μM, 100 μM Incubation Duration: 72 hrs (hours) Experimental Results: In K562 WT cells, the parental compound O4I2 demonstrated a marginal anti-proliferation effect (IC50 >10 μM). In In contrast, PROTAC-O4I2 demonstrated Dramatically higher toxicity with an IC50 of 228 nM, nearly 3-fold less potent than pladienolide B (IC50, 76 nM). Cells overexpressing SF3B1 WT was slightly resistant to pladienolide B (IC50, 134 nM), but more sensitive to PROTAC-O4I2 (an IC50 value of 63 nM). Apoptosis Analysis[1] Cell Types: K562 cell Tested Concentrations: 1 μM Incubation Duration: 48 h Experimental Results: Induced cellular apoptosis s in cells expressing SF3B1WT or SF3B1K700E. |
| Animal Protocol |
Animal/Disease Models: Drosophila melanogaster[1] Doses: 10 μM Route of Administration: Flies were fed on a round filter paper loaded with PROTAC-O4I2 in a 5% sucrose solution , maintained at 18℃ and flipped into a freshly prepared vial every 2 days. Experimental Results: diminished stem cell activity, blocked the initiation and growth of tumor, and improved the survival of the Drosophila ISC tumor model. |
| References |
[1]. A PROTAC targets splicing factor 3B1. Cell Chem Biol. 2021 Nov 18;28(11):1616-1627.e8. |
Solubility Data
| Solubility (In Vitro) | DMSO : 250 mg/mL (410.44 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.41 mM) (saturation unknown) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 + to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6418 mL | 8.2088 mL | 16.4177 mL | |
| 5 mM | 0.3284 mL | 1.6418 mL | 3.2835 mL | |
| 10 mM | 0.1642 mL | 0.8209 mL | 1.6418 mL |