PBTZ169 (PBTZ-169; Macozinone, an 8-Nitro-benzothiazinones (BTZs) analog) is a novel inhibitor of decaprenyl-phosphoribose-epimerase (DprE1) that displays nanomolar bactericidal activity against Mycobacterium tuberculosis in vitro. DprE1 is an essential enzyme involved in the cell wall biosynthesis of Corynebacterineae. Structure-activity relationship (SAR) studies revealed the 8-nitro group of the BTZ scaffold to be crucial for the mechanism of action, which involves formation of a semimercaptal bond with Cys387 in the active site of DprE1. When tested against thirty Nocardia brasiliensis isolates, the MIC50 and MIC90 values for PBTZ169 were 0.0075 and 0.03 μg/mL, respectively. Because Nocardia is a potential intracellular bacterium, a THP-1 macrophage monolayer was infected with N. brasiliensis HUJEG-1 and then treated with PBTZ169, resulting in a decrease in the number of colony-forming units (CFUs) at a concentration of 0.25X the in vitro value. The in vivo activity was evaluated after infecting female BALB/c mice in the right hind food-pad. After 6 weeks, treatment was initiated with PBTZ169 and its activity was compared with the first generation compound, BTZ043. Both BTZ compounds were administered at 100 mg/kg twice daily by gavage, and sulfamethoxazole/trimethoprim (SXT), at 100 mg/kg sulfamethoxazole, was used as a positive control. After 22 weeks of therapy, only PBTZ169 and SXT displayed statistically significant activity.
Physicochemical Properties
| Molecular Formula | C20H23F3N4O3S | |
| Molecular Weight | 456.48 | |
| Exact Mass | 456.144 | |
| Elemental Analysis | C, 52.62; H, 5.08; F, 12.49; N, 12.27; O, 10.51; S, 7.02 | |
| CAS # | 1377239-83-2 | |
| Related CAS # |
|
|
| PubChem CID | 57331386 | |
| Appearance | Solid powder | |
| Density | 1.5±0.1 g/cm3 | |
| Boiling Point | 555.6±60.0 °C at 760 mmHg | |
| Flash Point | 289.8±32.9 °C | |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C | |
| Index of Refraction | 1.660 | |
| LogP | 3.83 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 8 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 31 | |
| Complexity | 715 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O=C1C2=CC(C(F)(F)F)=CC([N+]([O-])=O)=C2SC(N3CCN(CC4CCCCC4)CC3)=N1 |
|
| InChi Key | BJDZBXGJNBMCAV-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C20H23F3N4O3S/c21-20(22,23)14-10-15-17(16(11-14)27(29)30)31-19(24-18(15)28)26-8-6-25(7-9-26)12-13-4-2-1-3-5-13/h10-11,13H,1-9,12H2 | |
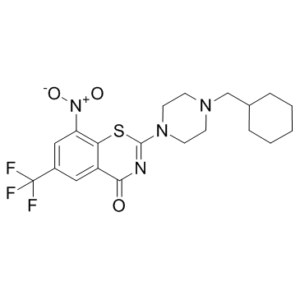
| Chemical Name | 2-(4-(cyclohexylmethyl)piperazin-1-yl)-8-nitro-6-(trifluoromethyl)-4H-benzo[e][1,3]thiazin-4-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | DprE1 | ||
| ln Vitro | PBTZ169 (also known as Macozinone, an 8-Nitro-benzothiazinones (BTZs) analog) is a novel inhibitor of decaprenyl-phosphoribose-epimerase (DprE1) that displays nanomolar bactericidal activity against Mycobacterium tuberculosis in vitro. DprE1 is an essential enzyme involved in the cell wall biosynthesis of Corynebacterineae. Structure-activity relationship (SAR) studies revealed the 8-nitro group of the BTZ scaffold to be crucial for the mechanism of action, which involves formation of a semimercaptal bond with Cys387 in the active site of DprE1. When tested against thirty Nocardia brasiliensis isolates, the MIC50 and MIC90 values for PBTZ169 were 0.0075 and 0.03 μg/mL, respectively. Because Nocardia is a potential intracellular bacterium, a THP-1 macrophage monolayer was infected with N. brasiliensis HUJEG-1 and then treated with PBTZ169, resulting in a decrease in the number of colony-forming units (CFUs) at a concentration of 0.25X the in vitro value. The in vivo activity was evaluated after infecting female BALB/c mice in the right hind food-pad. After 6 weeks, treatment was initiated with PBTZ169 and its activity was compared with the first generation compound, BTZ043. Both BTZ compounds were administered at 100 mg/kg twice daily by gavage, and sulfamethoxazole/trimethoprim (SXT), at 100 mg/kg sulfamethoxazole, was used as a positive control. After 22 weeks of therapy, only PBTZ169 and SXT displayed statistically significant activity. | ||
| ln Vivo |
|
||
| Enzyme Assay | PBTZ169, inhibit decaprenylphosphoryl-β-d-ribose 2′-oxidase (DprE1) and display nanomolar bactericidal activity against Mycobacterium tuberculosis in vitro. | ||
| Cell Assay | When tested against thirty Nocardia brasiliensis isolates, the MIC50 and MIC90 values for PBTZ169 were 0.0075 and 0.03 μg/mL, respectively. Because Nocardia is a potential intracellular bacterium, a THP-1 macrophage monolayer was infected with N. brasiliensis HUJEG-1 and then treated with PBTZ169, resulting in a decrease in the number of colony-forming units (CFUs) at a concentration of 0.25X the in vitro value. | ||
| Animal Protocol |
|
||
| References |
[1]. Antimicrob Agents Chemother.2015 Aug;59(8):4446-52. [2]. PLoS Negl Trop Dis.2015 Oct 16;9(10):e0004022. [3]. EMBO Mol Med. 2014 Mar;6(3):372-83.. |
||
| Additional Infomation | Macozinone is under investigation in clinical trial NCT03036163 (Phase 1 Study of PBTZ169). |
Solubility Data
| Solubility (In Vitro) |
DMSO: 5~6.4 mg/mL ( 10.95~14.02 mM) Water: <4 mg/mL |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1907 mL | 10.9534 mL | 21.9068 mL | |
| 5 mM | 0.4381 mL | 2.1907 mL | 4.3814 mL | |
| 10 mM | 0.2191 mL | 1.0953 mL | 2.1907 mL |