Physicochemical Properties
| Molecular Formula | C13H16N4O |
| Molecular Weight | 244.29 |
| Exact Mass | 252.195 |
| CAS # | 912444-01-0 |
| PubChem CID | 11842604 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 466.4±45.0 °C at 760 mmHg |
| Flash Point | 235.9±28.7 °C |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.522 |
| LogP | -0.88 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 18 |
| Complexity | 348 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | Cl.Cl.O=C(C1=CC=CC2=C1N=C([C@]1(C)CCCN1)N2)N |
| InChi Key | JNAHVYVRKWKWKQ-ZDUSSCGKSA-N |
| InChi Code | InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m0/s1 |
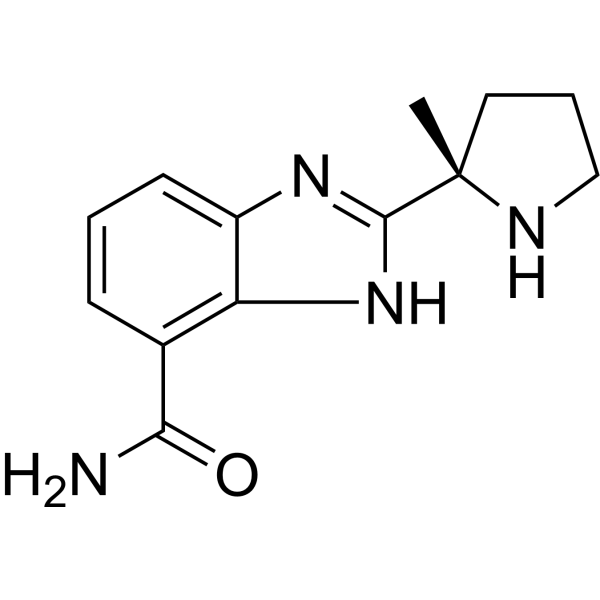
| Chemical Name | 2-[(2S)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide |
| Synonyms | 912444-01-0; 2-[(2s)-2-Methylpyrrolidin-2-Yl]-1h-Benzimidazole-7-Carboxamide; PARP-2/1-IN-2; 2-[(2S)-2-Methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide; 2-[(2S)-2-Methyl-2-pyrrolidinyl]-1H-benzimidazole-7-carboxamide; (R)-Modafinil-d10 Carboxylate Methyl Ester; (S)-2-(2-Methylpyrrolidin-2-yl)-1H-benzo[d]imidazole-4-carboxamide; 1217748-02-1; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PARP2 ( Ki = 2 nM ); PARP1 ( Ki = 5 nM ) |
| ln Vivo | PARP-2/1-IN-2 (Compound 4a) (25 or 50 mg/kg; ip) attenuates pain in cisplatin- and oxaliplatin-induced nerve damage in rats [1]. |
| Animal Protocol |
Animal/Disease Models: Male C57BL6J mouse, cisplatin- and oxaliplatin-induced painful neuropathy model [1] Doses: 25 mg/kg or 50 mg/kg Route of Administration: intraperitoneally (ip) (ip) two days before cisplatin or oxaliplatin treatment injection, continued intraperitonealdosing with concurrent cisplatin or oxaliplatin regimen (5 days followed by 5 days off, two weekly cycles) Experimental Results: Weight loss induced by cisplatin and oxaliplatin was not attenuated. The decrease in exploratory behavior associated with cisplatin and oxaliplatin treatment was not affected. Reduction of mechanical allodynia in cisplatin- and oxaliplatin-induced neuropathy. Alleviation of thermal hyperalgesia in cisplatin-induced neuropathy. Reduces cold hyperalgesia associated with oxaliplatin-induced neuropathy. |
| References |
[1]. A novel and selective poly (ADP-ribose) polymerase inhibitor ameliorates chemotherapy-induced painful neuropathy. PLoS One. 2013;8(1):e54161. |
| Additional Infomation | Chemotherapy-induced neuropathy is the principle dose limiting factor requiring discontinuation of many chemotherapeutic agents, including cisplatin and oxaliplatin. About 30 to 40% of patients receiving chemotherapy develop pain and sensory changes. Given that poly (ADP-ribose) polymerase (PARP) inhibition has been shown to provide neuroprotection, the current study was developed to test whether the novel PARP inhibitor compound 4a (analog of ABT-888) would attenuate pain in cisplatin and oxaliplatin-induced neuropathy in mice. Results: An established chemotherapy-induced painful neuropathy model of two weekly cycles of 10 intraperitoneal (i.p.) injections separated by 5 days rest was used to examine the therapeutic potential of the PARP inhibitor compound 4a. Behavioral testing using von Frey, paw radiant heat, cold plate, and exploratory behaviors were taken at baseline, and followed by testing at 3, 6, and 8 weeks from the beginning of drug treatment. Conclusion: Cisplatin-treated mice developed heat hyperalgesia and mechanical allodynia while oxaliplatin-treated mice exhibited cold hyperalgesia and mechanical allodynia. Co-administration of 50 mg/kg or 25 mg/kg compound 4a with platinum regimen, attenuated cisplatin-induced heat hyperalgesia and mechanical allodynia in a dose dependent manner. Similarly, co-administration of 50 mg/kg compound 4a attenuated oxaliplatin-induced cold hyperalgesia and mechanical allodynia. These data indicate that administration of a novel PARP inhibitor may have important applications as a therapeutic agent for human chemotherapy-induced painful neuropathy.[1] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0935 mL | 20.4675 mL | 40.9350 mL | |
| 5 mM | 0.8187 mL | 4.0935 mL | 8.1870 mL | |
| 10 mM | 0.4093 mL | 2.0467 mL | 4.0935 mL |