Physicochemical Properties
| Molecular Formula | C13H9N3O |
| Molecular Weight | 223.23 |
| Exact Mass | 223.075 |
| CAS # | 550-89-0 |
| PubChem CID | 120282 |
| Appearance | Light yellow to brown solid powder |
| Density | 1.371g/cm3 |
| Boiling Point | 526.1ºC at 760 mmHg |
| Melting Point | 242ºC |
| Flash Point | 272ºC |
| Index of Refraction | 1.76 |
| LogP | 2.582 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 17 |
| Complexity | 307 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | KPZYYKDXZKFBQU-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C13H9N3O/c14-13(17)8-4-3-7-11-12(8)16-10-6-2-1-5-9(10)15-11/h1-7H,(H2,14,17) |
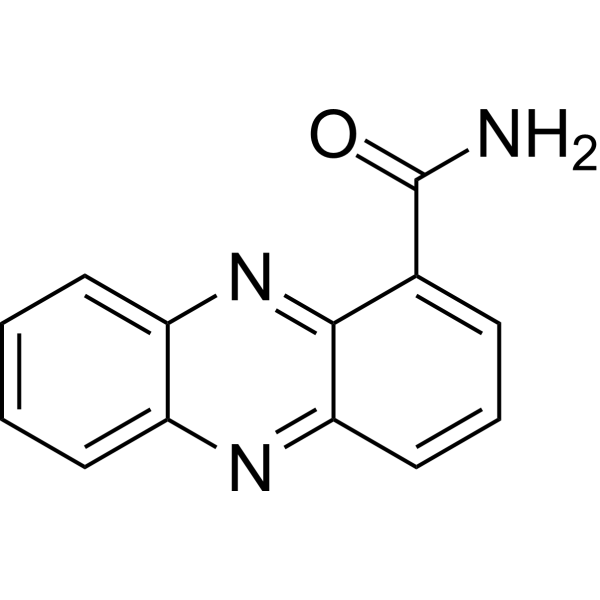
| Chemical Name | phenazine-1-carboxamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Oxychloroaphine (1-256 μM; 24 h) induces damage to cell membranes, increasing apoptosis and lactate dehydrogenase leakage, as well as increasing the formation of cytochrome c protein. The IC50 values for cytotoxic agents against A549, HeLa, and SW480 cancer cell lines range from 32 to 40 μM[2]. Cycle arrest at G1 phase and induction of sub-G phase are caused by oxychloroaphine (32 μM; A549 and SW480 cells) [2]. After 48 hours, oxychloroaphine (A549 cells) causes the proapoptotic protein caspase-3 to become activated, which in turn causes the cleavage of PARP[2]. This results in the downregulation of the antiapoptotic Bcl-2 protein. |
| Cell Assay |
Cell Viability Assay[2] Cell Types: A549, HeLa, and SW480 cancer cell lines Tested Concentrations: 1, 2, 4, 8, 16, 32, 64, 128, and 256 μM Incubation Duration: 24 hrs (hours) Experimental Results: Inhibited cell proliferative in a dose-dependent manner. |
| References |
[1]. Comparative metabolomics and transcriptomics analyses provide insights into the high-yield mechanism of phenazines biosynthesis in Pseudomonas chlororaphis GP72. J Appl Microbiol. 2022 Nov;133(5):2790-2801. [2]. Isolation of Bioactive Phenazine-1-Carboxamide from the Soil Bacterium Pantoea agglomerans and Study of Its Anticancer Potency on Different Cancer Cell Lines. J AOAC Int. 2016 Sep;99(5):1233-9. |
| Additional Infomation |
Phenazine-1-carboxamide is an aromatic amide that is phenazine substituted at C-1 with a carbamoyl group. It is a member of phenazines, an aromatic amide and a monocarboxylic acid amide. Phenazine-1-carboxamide has been reported in Streptomyces with data available. |
Solubility Data
| Solubility (In Vitro) | DMSO : 10 mg/mL (44.80 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.4797 mL | 22.3984 mL | 44.7968 mL | |
| 5 mM | 0.8959 mL | 4.4797 mL | 8.9594 mL | |
| 10 mM | 0.4480 mL | 2.2398 mL | 4.4797 mL |