Physicochemical Properties
| Molecular Formula | C19H18N3NAO5S |
| Molecular Weight | 423.4188 |
| Exact Mass | 423.086 |
| Elemental Analysis | C, 53.90; H, 4.29; N, 9.92; Na, 5.43; O, 18.89; S, 7.57 |
| CAS # | 1173-88-2 |
| Related CAS # | Oxacillin sodium monohydrate;7240-38-2;Oxacillin;66-79-5;Oxacillin-13C6 sodium |
| PubChem CID | 6196 |
| Appearance | Solid powder |
| Density | 1.49g/cm3 |
| Boiling Point | 686.8ºC at 760 mmHg |
| Melting Point | 188ºC |
| Flash Point | 369.2ºC |
| Vapour Pressure | 8.32E-20mmHg at 25°C |
| LogP | 0.889 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 28 |
| Complexity | 681 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | S1C(C([H])([H])[H])(C([H])([H])[H])[C@]([H])(C(=O)O[H])N2C([C@]([H])([C@@]12[H])/N=C(/C1=C(C([H])([H])[H])ON=C1C1C([H])=C([H])C([H])=C([H])C=1[H])\[O-])=O.[Na+] |
| InChi Key | VDUVBBMAXXHEQP-SLINCCQESA-M |
| InChi Code | InChI=1S/C19H19N3O5S.Na/c1-9-11(12(21-27-9)10-7-5-4-6-8-10)15(23)20-13-16(24)22-14(18(25)26)19(2,3)28-17(13)22/h4-8,13-14,17H,1-3H3,(H,20,23)(H,25,26)/q+1/p-1/t13-,14+,17-/m1./s1 |
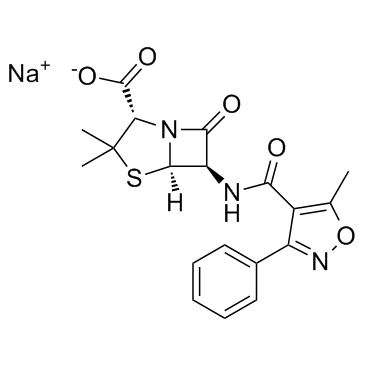
| Chemical Name | sodium (2S,5R,6R)-3,3-dimethyl-6-[(5-methyl-3-phenyl-1,2-oxazole-4-carbonyl)amino]-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate |
| Synonyms | Oxazocilline;Bactocill,Oxacillin;Oxacillin Sodium;Prostaphlin |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro | The MICs of 0.05, 0.09, 0.32, and 0.80 μg/mL for group A streptococci, pneumotocci, susceptible staphylococci, and penicillin-resistant staphylococci, respectively, indicate that oxacillin inhibits gram positive pathogens.Other penicillins are severely resistant to the strains that are resistant to oxacillin[1]. |
| ln Vivo | Mice infected with Staphylococcus aureus Evans exhibit a curative dose (CD50) of 253.3 mg/kg when treated with oxacillin (50-800 mg/kg; s.c.; once). Oxacillin's oral CD50 is 187.2 mg/kg[2]. |
| Animal Protocol |
Animal Model: S. aureus-infected male albino mice of the CD-1 strain Evans[2] Dosage: 50, 100, 200, 400 and 800 mg/kg Administration: Subcutaneous injection, once Result: demonstrated therapeutic efficacy at a 253.3 mg/kg CD50. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Oxacillin Sodium is rapidly excreted as unchanged drug in the urine by glomerular filtration and active tubular secretion. Biological Half-Life 20 to 30 minutes |
| Toxicity/Toxicokinetics |
Hepatotoxicity Oxacillin has been linked to two forms of hepatotoxicity, first an acute and transient elevation in serum aminotransferase levels occurring with high doses of intravenous therapy; and second, a more prolonged, usually cholestatic, idiosyncratic liver injury that is similar to the hepatotoxicity of other second-generation penicillins such as dicloxacillin, flucloxacillin, and nafcillin. High doses of intravenous oxacillin are commonly accompanied by elevations in serum ALT in the range of 2 to 20 times the upper limit of normal arising after 1 to 3 weeks of therapy. Alkaline phosphatase levels are only minimally elevated. Fever and nonspecific symptoms of abdominal pain and nausea can occur, but are often absent. Eosinophilia is present in some patients, but rash and arthralgias are uncommon. Serum aminotransferase levels rapidly fall into the normal range (in 1 to 2 weeks) with discontinuation of oxacillin or switch to lower doses, particularly in oral formulations. Jaundice does not occur. There appears to be no cross reactivity of this response with the natural penicillins, clindamycin or even nafcillin. Intravenous carbenicillin can cause a similar syndrome. This hepatotoxicity may be more common in HIV-positive than noninfected individuals. In addition to the common syndrome of asymptomatic serum aminotransferase elevations during high dose intravenous therapy, oxacillin can also but rarely lead to a more prolonged usually cholestatic hepatitis that appears 1 to 6 weeks after starting therapy and may persist for weeks to months. This form of idiosyncratic liver injury is similar to that described with dicloxacillin and other second generation penicillins. Immunoallergic features of rash, fever and eosinophilia can occur, but are not prominent. Autoantibodies are not found. The liver injury can be prolonged, but generally resolves within 1 to 2 months of onset. Liver biopsy generally shows a cholestatic hepatitis with mixed inflammatory infiltrates. Likelihood score: B (likely rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that oxacillin produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with penicillins, but these effects have not been adequately evaluated. Oxacillin is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 94.2 +/- 2.1% (binds to serum protein, mainly albumin) |
| References |
[1]. Oxacillin: laboratory and clinical evaluation. JAMA. 1962 Sep 1;181:739-44. [2]. Nafcillin and oxacillin: comparative antistaphylococcal activity in mice. J Antibiot (Tokyo). 1976 Apr;29(4):460-5. |
| Additional Infomation |
Oxacillin is a penicillin antibiotic carrying a 5-methyl-3-phenylisoxazole-4-carboxamide group at position 6beta. It has a role as an antibacterial agent and an antibacterial drug. It is a conjugate acid of an oxacillin(1-). An antibiotic similar to [flucloxacillin] used in resistant staphylococci infections. Oxacillin is a Penicillin-class Antibacterial. Oxacillin is a parenteral, second generation penicillin antibiotic that is used to treat moderate-to-severe, penicillinase-resistant staphylococcal infections. Oxacillin has been linked to rare instances of clinically apparent, idiosyncratic liver injury, but it more commonly causes transient elevations in serum aminotransferases without jaundice. Oxacillin has been reported in Bos taurus, Cordyceps farinosa, and Liquidambar formosana with data available. Oxacillin is a semisynthetic penicillinase-resistant and acid-stable penicillin with an antimicrobial activity. Oxacillin binds to penicillin-binding proteins in the bacterial cell wall, thereby blocking the synthesis of peptidoglycan, a critical component of the bacterial cell wall. This leads to inhibition of cell growth and causes cell lysis. Oxacillin Sodium is the sodium salt form of oxacillin, a semisynthetic penicillinase-resistant and acid-stable penicillin with an antimicrobial activity. Oxacillin binds to penicillin-binding proteins in the bacterial cell wall, thereby blocking the synthesis of peptidoglycan, a critical component of the bacterial cell wall. This leads to inhibition of cell growth and causes cell lysis. An antibiotic similar to FLUCLOXACILLIN used in resistant staphylococci infections. See also: Oxacillin Sodium (has salt form); Oxacillin benzathine (is active moiety of). Drug Indication Used in the treatment of resistant staphylococci infections. Mechanism of Action By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, Oxacillin inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that Oxacillin interferes with an autolysin inhibitor. Pharmacodynamics Oxacillin is a penicillin beta-lactam antibiotic used in the treatment of bacterial infections caused by susceptible, usually gram-positive, organisms. The name "penicillin" can either refer to several variants of penicillin available, or to the group of antibiotics derived from the penicillins. Oxacillin has in vitro activity against gram-positive and gram-negative aerobic and anaerobic bacteria. The bactericidal activity of Oxacillin results from the inhibition of cell wall synthesis and is mediated through Oxacillin binding to penicillin binding proteins (PBPs). Oxacillin is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, and cephalosporinases and extended spectrum beta-lactamases. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 85~125 mg/mL ( 200.74~295.22 mM ) Water : ~85 mg/mL Ethanol : ~8 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.90 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.90 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.90 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (5.90 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3617 mL | 11.8086 mL | 23.6172 mL | |
| 5 mM | 0.4723 mL | 2.3617 mL | 4.7234 mL | |
| 10 mM | 0.2362 mL | 1.1809 mL | 2.3617 mL |