Physicochemical Properties
| Molecular Formula | C18H30N2O5 |
| Molecular Weight | 354.441205501556 |
| Exact Mass | 354.215 |
| CAS # | 1191921-01-3 |
| PubChem CID | 146065662 |
| Appearance | White to off-white solid powder |
| LogP | 1.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 25 |
| Complexity | 514 |
| Defined Atom Stereocenter Count | 3 |
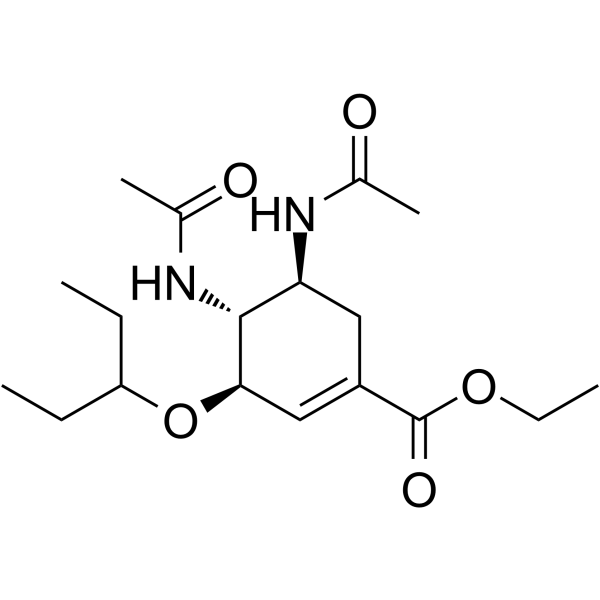
| SMILES | O(C(CC)CC)C1C=C(C(=O)OCC)CC(C1NC(C)=O)NC(C)=O |
| InChi Key | IWASCEKXRLUKKH-GVDBMIGSSA-N |
| InChi Code | InChI=1S/C18H30N2O5/c1-6-14(7-2)25-16-10-13(18(23)24-8-3)9-15(19-11(4)21)17(16)20-12(5)22/h10,14-17H,6-9H2,1-5H3,(H,19,21)(H,20,22)/t15-,16+,17+/m0/s1 |
| Chemical Name | ethyl (3R,4R,5S)-4,5-diacetamido-3-pentan-3-yloxycyclohexene-1-carboxylate |
| Synonyms | Oseltamivir-acetate; 1191921-01-3; Ethyl (3R,4R,5S)-4,5-diacetamido-3-pentan-3-yloxycyclohexene-1-carboxylate; (3R,4R,5S)-Ethyl 4,5-diacetamido-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Oseltamivir impurity |
| References |
[1]. Transplacental transfer of Oseltamivir phosphate and its metabolite Oseltamivir carboxylate using the ex vivo human placenta perfusion model in Chinese Hans population. J Matern Fetal Neonatal Med. 2016 Aug 8:1-5. [2]. Anti-influenza neuraminidase inhibitor Oseltamivir phosphate induces canine mammary cancer cell aggressiveness. PLoS One. 2015 Apr 7;10(4):e0121590. |
| Additional Infomation |
Objective: The objective of the study was to determine transplacental of oseltamivir phosphate (OP) and its active metabolite oseltamivir carboxylate (OC) using the ex vivo human placental perfusion model in Chinese Hans population.
Study design: Perfusion studies were performed on 20 placentas from healthy term pregnancies. Concentrations typical for 75 mg, twice-daily oral dose were tested (OP 65.2 ng/ml and OC 348 ng/ml), along with the positive control antipyrine 0.1 mg/ml. Each perfusion experiment was conducted for 180 min while samples were taken from both maternal and fetal compartments. Concentrations of OP and its metabolite OC were determined by ultrafast-performance liquid chromatography/tandem mass spectrometry. Integrity and viability of the placenta were determined by measuring fetal volume loss, pH, pO2, ΔhCG, glucose consumption and lactate production during the perfusion experiments.
Results: Following 3 h of perfusion, the fetal transfer rates of OP and its metabolite OC were 12.39%±3.26%, 10.17%±2.03%, respectively. The clearance indexes of OP and OC were 0.36 ± 0.11 and 0.29 ± 0.06, respectively.
Conclusion: These data suggest that OP and its metabolite OC pass through the healthy term placenta at a small amount according to the ex vivo human placental perfusion model, fetal exposure must be considered when treating pregnant women with OP.[1] Oseltamivir phosphate is a widely used anti-influenza sialidase inhibitor. Sialylation, governed by sialyltransferases and sialidases, is strongly implicated in the oncogenesis and progression of breast cancer. In this study we evaluated the biological behavior of canine mammary tumor cells upon oseltamivir phosphate treatment (a sialidase inhibitor) in vitro and in vivo. Our in vitro results showed that oseltamivir phosphate impairs sialidase activity leading to increased sialylation in CMA07 and CMT-U27 canine mammary cancer cells. Surprisingly, oseltamivir phosphate stimulated, CMT-U27 cell migration and invasion capacity in vitro, in a dose-dependent manner. CMT-U27 tumors xenograft of oseltamivir phosphate-treated nude mice showed increased sialylation, namely α2,6 terminal structures and SLe(x) expression. Remarkably, a trend towards increased lung metastases was observed in oseltamivir phosphate-treated nude mice. Taken together, our findings revealed that oseltamivir impairs canine mammary cancer cell sialidase activity, altering the sialylation pattern of canine mammary tumors, and leading, surprisingly, to in vitro and in vivo increased mammary tumor aggressiveness.[2] |
Solubility Data
| Solubility (In Vitro) | DMSO : 50 mg/mL (141.07 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.05 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.05 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.05 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8214 mL | 14.1068 mL | 28.2135 mL | |
| 5 mM | 0.5643 mL | 2.8214 mL | 5.6427 mL | |
| 10 mM | 0.2821 mL | 1.4107 mL | 2.8214 mL |