Physicochemical Properties
| Molecular Formula | C8H16O2 |
| Molecular Weight | 144.21 |
| Exact Mass | 144.115 |
| Elemental Analysis | C, 66.63; H, 11.18; O, 22.19 |
| CAS # | 124-07-2 |
| Related CAS # | 15696-43-2 (unspecified lead salt);16577-52-9 (lithium salt);18312-04-4 (unspecified zirconium salt);1912-83-0 (tin(+2) salt);1984-06-1 (hydrochloride salt);20195-23-7 (unspecified chromium salt);20543-04-8 (unspecified copper salt);2191-10-8 (cadmium salt);3130-28-7 (iron(+3) salt);3890-89-9 (copper(+2) salt);4696-54-2 (barium salt);4995-91-9 (nickel(+2) salt);5206-47-3 (zirconium(+4) salt);557-09-5 (zinc salt);5972-76-9 (ammonium salt);6028-57-5 (aluminum salt);60903-69-7 (La(+3) salt);6107-56-8 (calcium salt);6427-90-3 (chromium(+2) salt);6535-19-9 (unspecified manganese salt);6535-20-2 (unspecified iron salt);6700-85-2 (cobalt salt);67816-08-4 (Ir(+3) salt);68957-64-2 (Ru(+3) salt);7319-86-0 (lead(+2) salt);7435-02-1 (unspecified Ce salt);764-71-6 (potassium salt) |
| PubChem CID | 379 |
| Appearance | Colorless to light yellow liquid |
| Density | 0.91 g/mL at 25 °C(lit.) |
| Boiling Point | 237 ºC |
| Melting Point | 16 °C |
| Flash Point | 130 ºC |
| Vapour Pressure | 0.022mmHg at 25°C |
| Index of Refraction | n20/D 1.428(lit.) |
| LogP | 2.431 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 10 |
| Complexity | 89.3 |
| Defined Atom Stereocenter Count | 0 |
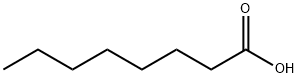
| SMILES | CCCCCCCC(O)=O |
| InChi Key | WWZKQHOCKIZLMA-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C8H16O2/c1-2-3-4-5-6-7-8(9)10/h2-7H2,1H3,(H,9,10) |
| Chemical Name | octanoic acid |
| Synonyms | octanoic acid; caprylic acid; 124-07-2; n-octanoic acid; Octylic acid; n-caprylic acid; octoic acid; n-octylic acid; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets |
- Branched-chain alpha-keto acid dehydrogenase kinase (BCKDK)
- Octanoic acid inhibits hepatic BCKDK (a negative regulator of branched-chain alpha-keto acid dehydrogenase, BCKDH), but no specific IC50 or Ki values were reported [3] - Olfactory receptor 51E2 (OR51E2) - Octanoic acid binds to OR51E2 in pancreatic β-cells to modulate insulin secretion, with no measured binding affinity (KD) or EC50 values provided [4] |
| ln Vitro |
- Neurotrophic effects (PC12 cells): 1. Neurite outgrowth promotion: Octanoic acid (100–400 μM) treatment for 72 hours increased neurite length by 2.0–3.5-fold and neurite-bearing cell ratio by 40–60% in PC12 cells (compared to vehicle control). At 200 μM, it induced maximal neurite outgrowth, comparable to nerve growth factor (NGF, 50 ng/mL) [5] 2. Signaling activation: Octanoic acid (100–400 μM) increased phosphorylation of ERK1/2 (1.5–2.5-fold) and Akt (1.2–1.8-fold) in PC12 cells, as detected by western blot. Inhibition of ERK pathway (U0126, 10 μM) abolished neurite outgrowth induced by octanoic acid [5] - Insulin secretion modulation (pancreatic β-cells): 1. Glucose-stimulated insulin secretion (GSIS) enhancement: In INS-1 rat insulinoma cells, octanoic acid (100–500 μM) potentiated GSIS (16.7 mM glucose) by 1.5–2.2-fold. This effect was abolished by OR51E2 siRNA transfection, indicating OR51E2 dependence [4] 2. Glucokinase (GK) upregulation: octanoic acid (200 μM) increased GK mRNA expression by 1.8-fold and GK protein levels by 1.6-fold in INS-1 cells (qPCR and western blot analysis) [4] - Branched-chain amino acid (BCAA) catabolism regulation (primary hepatocytes): 1. BCKDH activation: Octanoic acid (100–500 μM) treatment for 4 hours increased hepatic BCKDH activity by 1.3–2.0-fold in rat primary hepatocytes. This was associated with reduced BCKDK protein levels (by 30–50%) [3] 2. BCAA degradation promotion: Octanoic acid (300 μM) increased the degradation rate of [14C]-labeled leucine (a BCAA) by 40% in primary hepatocytes [3] |
| ln Vivo |
- Tremor suppression (essential tremor mouse model): 1. Harmaline-induced tremor attenuation: Male ICR mice (25–30 g) received octanoic acid (100 or 200 mg/kg, ip) 30 minutes before harmaline (50 mg/kg, ip). At 200 mg/kg, octanoic acid reduced tremor score (0–3 scale) from 2.8 (vehicle) to 1.2 and decreased tremor frequency (Hz) by 35% (measured by accelerometer and visual scoring) [1] 2. Duration of effect: The tremor-suppressive effect lasted for 2–3 hours post-administration [1] - Parkinson’s disease protection (MPTP mouse model): 1. Dopamine preservation: Male C57BL/6 mice (20–25 g) received octanoic acid (100 mg/kg, ip) daily for 5 days, with MPTP (20 mg/kg, ip) administered on days 2–4. Octanoic acid prevented MPTP-induced striatal dopamine (DA) reduction: DA levels were 85% of control (vs. 40% in MPTP-only group) as measured by HPLC [2] 2. Motor function improvement: Octanoic acid reversed MPTP-induced deficits in rotarod performance (latency to fall increased from 40 s to 80 s) and open-field locomotor activity (distance traveled increased by 50%) [2] - BCAA metabolism regulation (rat model): 1. Plasma BCAA reduction: Male Sprague-Dawley rats (250–300 g) were orally administered octanoic acid (200 or 400 mg/kg) daily for 7 days. At 400 mg/kg, plasma leucine, isoleucine, and valine concentrations decreased by 30%, 25%, and 28%, respectively [3] 2. Hepatic BCKDH activation: Octanoic acid (400 mg/kg, oral) increased hepatic BCKDH activity by 60% and reduced hepatic BCKDK protein levels by 45% in rats [3] |
| Enzyme Assay |
- BCKDK inhibition assay (rat liver) [3]: 1. Enzyme extraction: Rat liver tissue was homogenized in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) and centrifuged at 12,000×g for 20 minutes to obtain BCKDK-containing supernatant. 2. Reaction setup: The assay mixture (200 μL) contained BCKDK extract (50 μg protein), BCKDH substrate (100 μM branched-chain alpha-keto acids), ATP (1 mM), MgCl2 (5 mM), and octanoic acid (0.1–1 mM, vehicle: ethanol). 3. Activity measurement: BCKDH activity (indicator of BCKDK inhibition) was measured by monitoring NADH production at 340 nm for 30 minutes. Relative activity was calculated compared to the vehicle control. - Insulin secretion assay (INS-1 cells) [4]: 1. Cell preparation: INS-1 cells were cultured in RPMI 1640 medium with 10% FBS and synchronized in glucose-free Krebs-Ringer bicarbonate buffer (KRBB) for 2 hours. 2. Stimulation and detection: Cells were treated with octanoic acid (100–500 μM) in KRBB containing 2.8 mM (low glucose) or 16.7 mM (high glucose) glucose for 1 hour. Insulin in the supernatant was quantified by ELISA, and GSIS was calculated as the ratio of insulin secretion under high vs. low glucose. |
| Cell Assay |
- PC12 cell neurite outgrowth assay [5]: 1. Cell culture: PC12 cells were seeded in 24-well plates (5×10⁴ cells/well) in DMEM with 10% FBS and 5% horse serum. 2. Drug treatment: After 24 hours, octanoic acid (100–400 μM, vehicle: ethanol) or NGF (50 ng/mL, positive control) was added, and cells were cultured for 72 hours. 3. Neurite quantification: Cells were fixed with 4% paraformaldehyde, stained with crystal violet, and observed under a light microscope. Neurite length and neurite-bearing cells were counted in 5 random fields per well; neurites longer than twice the cell body diameter were considered positive. 4. Signaling detection: For western blot, PC12 cells were lysed in RIPA buffer, and proteins (30 μg) were probed with anti-p-ERK1/2, anti-ERK1/2, anti-p-Akt, and anti-Akt antibodies. - Primary hepatocyte BCKDH activity assay [3]: 1. Hepatocyte isolation: Rat primary hepatocytes were isolated by collagenase perfusion and seeded in 6-well plates (1×10⁶ cells/well) in William’s E medium. 2. Drug treatment: Octanoic acid (100–500 μM) was added 24 hours post-seeding, and cells were incubated for 4 hours. 3. BCKDH activity measurement: Cells were homogenized, and BCKDH activity was assayed by monitoring NADH production at 340 nm after adding branched-chain alpha-keto acids and cofactors. |
| Animal Protocol |
- Mouse essential tremor model [1]: 1. Animal selection: Male ICR mice (25–30 g) were acclimated for 7 days before experimentation. 2. Drug formulation: Octanoic acid was dissolved in physiological saline (pH adjusted to 7.4 with NaOH) to concentrations of 10 mg/mL (100 mg/kg dose) and 20 mg/mL (200 mg/kg dose). 3. Administration: Mice received intraperitoneal (ip) injections of octanoic acid (10 mL/kg volume) 30 minutes before ip injection of harmaline (5 mg/mL in saline, 50 mg/kg). 4. Tremor assessment: Tremor was evaluated visually (score 0–3: 0=no tremor, 3=severe tremor) and by accelerometer (recording frequency and amplitude) at 15, 30, 60, 90, 120, and 180 minutes post-harmaline injection. - Mouse MPTP Parkinson’s model [2]: 1. Animal selection: Male C57BL/6 mice (20–25 g) were used. 2. Drug formulation: Octanoic acid was dissolved in saline (pH 7.4) to 10 mg/mL. 3. Administration schedule: Mice received daily ip injections of octanoic acid (100 mg/kg, 10 mL/kg) on days 1–5. MPTP (2 mg/mL in saline) was administered ip at 20 mg/kg on days 2–4 (once daily). 4. Endpoint measurements: On day 6, mice were euthanized; striatum was dissected for dopamine analysis (HPLC). Motor function was assessed by rotarod test (5 rpm acceleration) and open-field test (5-minute session) on day 5. - Rat BCAA metabolism model [3]: 1. Animal selection: Male Sprague-Dawley rats (250–300 g) were fasted overnight before experimentation. 2. Drug formulation: Octanoic acid was dissolved in corn oil to concentrations of 20 mg/mL (200 mg/kg dose) and 40 mg/mL (400 mg/kg dose). 3. Administration: Rats received oral gavage of octanoic acid (10 mL/kg volume) daily for 7 days. Control rats received corn oil alone. 4. Endpoint measurements: On day 8, blood was collected via cardiac puncture for plasma BCAA analysis (HPLC); liver tissue was harvested for BCKDH activity assay and BCKDK western blot. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Children who suffer from seizures which are not controllable by drugs have apparently been successfully treated with MCT (medium chain triglyceride) diet. The MCT diet is an emulsion containing primarily (81%) octanoic acid, but also contains 15% decanoic acid. In this study 15 children were receiving 50 to 60% of their energy requirement s from the MCT emulsion. Blood samples were analyzed for decanoic and octanoic acid levels. There was a wide variation in absolute levels, possibly due to poor patient compliance, but all patients showed low levels in the mornings, rising to high levels in the evenings. This suggested that both acids are rapidly metabolized. /Medium chain triglyceride/ To assess the disposition kinetics of selected structural analogs of valproic acid, the pharmacokinetics of valproic acid and 3 structural analogs, cyclohexanecarboxylic acid, l-methyl-l-cyclohexanecarboxylic acid (1-methylcyclohexanecarboxylic acid; and octanoic acid were examined in female rats. All 4 carboxylic acids evidenced dose-dependent disposition. A dose-related decrease in total body clearance was observed for each compound, suggesting saturable eiminination processes. The apparent volume of distribution for these compounds was, with the exception of cyclohexanecarboxylic acid, dose-dependent, indicating that binding to proteins in serum and/or tissues may be saturable. Both valproic acid and 1-methylcyclohexanecarboxylic acid exhibited enterohepatic recirculation, which appeared to be dose- and compound-dependent. Significant quantities of both valproic acid and 1-methylcyclohexanecarboxylic acid were excreted in the urine as conjugates. Octanoic acid and cyclohexanecarboxylic acid were not excreted in the urine and did not evidence enterohepatic recirculation. It was concluded that minor changes in chemical structure of low molecular weight carboxylic acids have an influence on their metabolism and disposition. Metabolism / Metabolites Caprylic acid administered to rats is readily metabolized by the liver and many other tissues, forming carbon dioxide and two-carbon fragments, which are incorporated into long-chain fatty acids, as well as other water-soluble products. The enzyme MCAD (medium-chain acyl-CoA dehydrogenase) is responsible for the dehydrogenation step of fatty acids with chain lengths between 6 and 12 carbons as they undergo beta-oxidation in the mitochondria. Fatty acid beta-oxidation provides energy after the body has used up its stores of glucose and glycogen. This typically occurs during periods of extended fasting or illness when caloric intake is reduced, and energy needs are increased. Beta-oxidation of long chain fatty acids produces two carbon units, acetyl-CoA and the reducing equivalents NADH and FADH2. NADH and FADH2 enter the electron transport chain and are used to make ATP. Acetyl-CoA enters the Krebs Cycle and is also used to make ATP via the electron transport chain and substrate level phosphorylation. When the supply of acetyl-CoA (coming from the beta-oxidation of fatty acids) exceeds the capacity of the Krebs Cycle to metabolize acetyl-CoA, the excess acetyl-CoA molecules are converted to ketone bodies (acetoacetate and beta-hydroxybutyrate) by HMG-CoA synthase in the liver. Ketone bodies can also be used for energy especially by the brain and heart; in fact they become the main sources of energy for those two organs after day three of starvation. (Wikipedia) - Oral absorption (rats): After oral administration of octanoic acid (400 mg/kg in corn oil), peak plasma concentration (Cmax) of 150–200 μM was reached at 1–2 hours (Tmax). Oral bioavailability was approximately 85–90% [3] - Distribution: Octanoic acid showed preferential distribution to the liver (tissue/plasma concentration ratio: 2.5–3.0) and pancreas (1.8–2.0) in rats. It crosses the blood-brain barrier, with brain concentrations reaching 30–40% of plasma levels [2,3] - Metabolism and excretion: Octanoic acid is rapidly metabolized via β-oxidation in the liver to acetyl-CoA (a substrate for the Krebs cycle). Approximately 90% of the administered dose is oxidized to CO2 and excreted via respiration within 24 hours; <5% is excreted unchanged in urine [3] - Elimination half-life: The plasma elimination half-life (t1/2) of octanoic acid in rats is 1.5–2.0 hours [3] |
| Toxicity/Toxicokinetics |
Toxicity Summary It has been demonstrated that octanoic (OA) and decanoic (DA) acids compromise the glycolytic pathway and citric acid cycle functioning, increase oxygen consumption in the liver and inhibit some activities of the respiratory chain complexes and creatine kinase in rat brain (A15454, A15455). These fatty acids were also shown to induce oxidative stress in the brain (A15456). Experiments suggest that OA and DA impair brain mitochondrial energy homeostasis that could underlie at least in part the neuropathology of MCADD. (A15457) Toxicity Data Oral rat LD50: 10080 mg/kg. Intravenous mouse LD50: 600 mg/kg. Skin rabbit LD50: over 5000 mg/kg. Interactions The duration of the absorption-enhancing effect of sodium octanoate (C8), sodium hexanoate (C6) and glyceryl-l-monooctanoate (MO) on the rectal absorption of gentamicin using the hollow-type suppository was investigated in rabbits. To evaluate the duration of the absorption-enhancing effect by pretreatment (treatment of absorption enhancer before gentamicin administration), suppository I containing each absorption enhancer in the cavity was administered into the rectum. Then suppository II containing gentamicin in the cavity was administered at predetermined times 10.33, 2, 6 and 24 hr after the administration of suppository I. Plasma gentamicin levels obtained by the pretreatment with absorption enhancer were compared with those obtained by the simultaneous administration of gentamicin with absorption enhancer. The AUC and Cmax of gentamicin significantly decreased with the pretreatment of C8-16 and 24 hr, C6-12 and 6 hr or glyceryl-l-monooctanoate 16 and 24 hr before rectal gentamicin administration, as compared with the simultaneous administration of gentamicin with C8, C6 or glyceryl-l-monooctanoate. A marked decrease in the absorption-enhancing effect of C8, C6 and glyceryl-l-monooctanoate on rectal gentamicin absorption was observed by the prolongation of the period between the pretreatment of each absorption enhancer and gentamicin administration. The duration of the absorption-enhancing effect of C6 was shorter than that of C8, whereas this duration of glyceryl-l-monooctanoate was similar to that of C8. The effect of these absorption enhancers disappeared 24 hr after the pretreatment. These results suggested that the lowering of the membrane transport barrier function recovered about one day after the administration of C8 or MO. Cytochrome oxidase activity was investigated histochemically in the choroid plexus epithelium. Intense staining for the enzyme was exclusively limited to the mitochondria. Rats treated with octanoic acid displayed extensive ultrastructural disruptions in the epithelial cells of the choroid plexus. Mitochondria were fewer in number and more disrupted compared to the control. The enzyme activity was greatly reduced. However, pretreatment with an equimolar dose of L-carnitine followed by octanoic acid injection produced little alteration of either ultrastructure or enzyme staining. This study suggests that L-carnitine supplementation may restore mitochondrial function of the choroid plexus subjected to toxic organic anions in metabolic disorders, and may be useful in the prevention of metabolic encephalopathy. The effects of sodium caprate and sodium caprylate on transcellular permeation routes were examined in rats. The release of membrane phospholipids was significantly increased only by caprate, while protein release did not change from the control in the presence of caprate or caprylate, indicating that the extent of membrane disruption was insufficient to account for the extent of the enhanced permeation. Using brush border membrane vesicles prepared from colon, with their protein and lipid component labeled by fluorescent probes, the perturbing actions of caprate and caprylate toward the membrane were examined by fluorescence polarization. Caprate interacted with membrane protein and lipids, and caprylate mainly with protein, causing perturbation to the membrane. The release of 5(6)-carboxyfluorescein previously included in brush border membrane vesicles was increased by caprate but not by caprylate ... /Sodium caprylate/ /In the/ Escherichia coli reverse mutation assay ... octanoic acid inhibited the mutagenic activity of N-nitrosodimethylamine in E. coli bacteria and the extent to which this mutagen methylated DNA in cultured calf thymus cells. Non-Human Toxicity Values LD50 Rat oral 1410 mg/kg LD50 Rat oral 14.7 mL/kg /C6 0.5%, C8 97.9%, C10 1.6%, C12 traces/ LD50 Rat gavage 1.41mL (1283 mg)/kg LD50 Rabbit dermal >5000 mg/kg LD50 Rabbit dermal 0.71 mL (647 mg)/kg - In vitro toxicity: 1. Cell viability: Octanoic acid (≤500 μM) showed no cytotoxicity in PC12 cells, INS-1 cells, or rat primary hepatocytes (MTT assay, cell viability >90% vs. control) [3,4,5] 2. pH effect: At concentrations >1 mM, octanoic acid caused mild acidification of cell culture media, but this was mitigated by pH adjustment of stock solutions [5] - In vivo toxicity: 1. Acute toxicity: In mice, ip LD50 of octanoic acid was >1000 mg/kg; in rats, oral LD50 was >2000 mg/kg. No mortality or overt toxicity (e.g., lethargy, diarrhea) was observed at doses ≤400 mg/kg [1,2,3] 2. Subchronic toxicity: Rats treated with octanoic acid (400 mg/kg, oral) daily for 28 days showed no significant changes in body weight, food intake, or serum markers of liver (ALT, AST) and kidney (creatinine, BUN) function [3] - Plasma protein binding: Octanoic acid has low plasma protein binding (15–20%) in rats and mice [3] |
| References |
[1]. Octanoic acid suppresses harmaline-induced tremor in mouse model of essential tremor. Neurotherapeutics. 2012 Jul;9(3):635-8. [2]. Octanoic acid prevents reduction of striatal dopamine in the MPTP mouse model of Parkinson's disease. Pharmacol Rep. 2018 Oct;70(5):988-992. [3]. Octanoic acid promotes branched-chain amino acid catabolisms via the inhibition of hepatic branched-chain alpha-keto acid dehydrogenase kinase in rats. Metabolism. 2015 Sep;64(9):1157-64. [4]. Octanoic acid potentiates glucose-stimulated insulin secretion and expression of glucokinase through the olfactory receptor in pancreatic β-cells. Biochem Biophys Res Commun. 2018 Sep 3;503(1):278-284. [5]. Induction of neurite outgrowth in PC12 cells by the medium-chain fatty acid octanoic acid. Neuroscience. 2007 May 25;146(3):1073-81. |
| Additional Infomation |
Therapeutic Uses Exptl Use: A simple methodology for hyperimmune horse plasma fractionation, based on caprylic acid precipitation, is described. Optimal conditions for fractionation were studied; the method gives best results when concentrated caprylic acid was added to plasma, whose pH had been adjusted to 5.8, until a final caprylic acid concentration of 5% was reached. The mixture was vigorously stirred during caprylic acid addition and then for 60 min; afterwards the mixture was filtered. Non-immunoglobulin proteins precipitated in these conditions, whereas a highly enriched immunoglobulin preparation was obtained in the filtrate, which was then dialysed to remove caprylic acid before the addition of sodium chloride and phenol. Thus, antivenon was produced after a single precipitation step followed by dialysis. In order to compare this methodology with that based on ammonium sulfate fractionation, a sample of hyperimmune plasma was divided into two aliquots which were fractionated in parallel by both methods. It was found that caprylic acid-fractionated antivenom was superior in terms of yield, production time, albumin/globulin ratio, turbidity, protein aggregates, electrophoretic pattern and neutralizing potency against several activities of Bothrops asper venom. Owing to its efficacy and simplicity, this method could be of great value in antivenom and antitoxin production laboratories. /EXPL THER/ The treatment for patients with genetic disorders of mitochondrial long-chain fatty acid beta-oxidation is directed toward providing sufficient sources of energy for normal growth and development, and at the same time preventing the adverse effects that precipitate or result from metabolic decompensation. Standard of care treatment has focused on preventing the mobilization of lipids that result from fasting and providing medium-chain triglycerides (MCT) in the diet in order to bypass the long-chain metabolic block. MCTs that are currently available as commercial preparations are in the form of even-chain fatty acids that are predominately a mixture of octanoate and decanoate ... The even-numbered medium-chain fatty acids (MCFAs) that are found in MCT preparations can reduce the accumulation of potentially toxic long-chain metabolites of fatty acid oxidation (FAO) ... /Octanoate/ Children who suffer from seizures which are not controllable by drugs have apparently been successfully treated with MCT (medium chain triglyceride) diet. The MCT diet is an emulsion containing primarily (81%) octanoic acid, but also contains 15% decanoic acid ... /Medium chain triglyceride/ Octanoic acid appears as a colorless to light yellow liquid with a mild odor. Burns, but may be difficult to ignite. Corrosive to metals and tissue. Octanoic acid is a straight-chain saturated fatty acid that is heptane in which one of the hydrogens of a terminal methyl group has been replaced by a carboxy group. Octanoic acid is also known as caprylic acid. It has a role as an antibacterial agent, a human metabolite and an Escherichia coli metabolite. It is a straight-chain saturated fatty acid and a medium-chain fatty acid. It is a conjugate acid of an octanoate. The CIR Expert Panel concluded that the following ingredients are safe in the present practices of use andconcentration described in the safety assessment when formulated to be non-irritating and non-sensitizing, which may be basedon a QRA...Caprylic Acid... Caprylic acid is an eight-carbon chain fatty acid, also known systematically as octanoic acid. It is found naturally in coconuts and breast milk. It is an oily liquid with a slightly unpleasant rancid-like smell that is minimally soluble in water. Caprylic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Octanoic acid has been reported in Alpinia latilabris, Artemisia xerophytica, and other organisms with data available. Octanoic Acid is a saturated medium-chain fatty acid with an 8-carbon backbone. Octanoic acid is found naturally in the milk of various mammals and is a minor component of coconut oil and palm kernel oil. OCTANOIC ACID is a small molecule drug with a maximum clinical trial phase of II (across all indications) and has 8 investigational indications. Caprylic acid is the common name for the eight-carbon straight chain fatty acid known by the systematic name octanoic acid. It is found naturally in coconuts and breast milk. It is an oily liquid with a slightly unpleasant rancid taste that is minimally soluble in water. Caprylic acid is used commercially in the production of esters used in perfumery and also in the manufacture of dyes. - Background: Octanoic acid is a naturally occurring medium-chain fatty acid (MCFA) found in coconut oil, palm kernel oil, and human milk. It is metabolized more rapidly than long-chain fatty acids, providing a quick source of energy [3,5] - Mechanistic diversity: Octanoic acid exerts multiple biological effects via distinct pathways: (1) neuroprotection through ERK/Akt signaling activation; (2) metabolic regulation via BCKDK inhibition and OR51E2-mediated insulin modulation; (3) tremor suppression via unclear mechanisms (possibly involving GABAergic or dopaminergic pathways) [1,3,4,5] - Therapeutic potential: Octanoic acid is being investigated for the treatment of neurological disorders (essential tremor, Parkinson’s disease) and metabolic diseases (insulin resistance, BCAA metabolic disorders). It is not currently approved by the FDA for these indications but is used as a dietary supplement [1,2,3,4] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.9343 mL | 34.6717 mL | 69.3433 mL | |
| 5 mM | 1.3869 mL | 6.9343 mL | 13.8687 mL | |
| 10 mM | 0.6934 mL | 3.4672 mL | 6.9343 mL |