ON123300 is a novel, low molecular weight and potent multikinase inhibitor identified through a series of screens that supported further analyses for brain tumor chemotherapy. It has minimal inhibitory action against other CDKs like 1, 2, 5, and 8, and inhibits CDK4 with an IC50 of 3.8 nM. PDGFRβ-type platelet-derived growth factor receptor (PDGFRβ) and CDK4, as well as growth factor receptor tyrosine kinases, were all strongly inhibited by ON123300, according to biochemical assays. ON123300 was found to inhibit the proliferation of U87 glioma cells with an IC(50) of 3.4 ± 0.1 μmol/L and to reduce Akt phosphorylation. However, it also surprisingly induced Erk activation in a dose- and time-dependent manner, which was later linked to the relief of Akt-mediated C-Raf S259 inactivation and the activation of a p70S6K-initiated PI3K-negative feedback loop. Since ON123300 showed good pharmacokinetic properties, combinations that reduce its Erk activation and increase its activity—like gefitinib—will likely be needed in its future development as a brain tumor treatment.
Physicochemical Properties
| Molecular Formula | C₂₄H₂₇N₇O | |
| Molecular Weight | 429.52 | |
| Exact Mass | 429.228 | |
| Elemental Analysis | C, 67.11; H, 6.34; N, 22.83; O, 3.72 | |
| CAS # | 1357470-29-1 | |
| Related CAS # |
|
|
| PubChem CID | 56649281 | |
| Appearance | Yellow to orange solid powder | |
| LogP | 3.349 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 32 | |
| Complexity | 754 | |
| Defined Atom Stereocenter Count | 0 | |
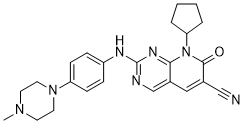
| SMILES | O=C1C(C#N)=C([H])C2=C([H])N=C(N([H])C3C([H])=C([H])C(=C([H])C=3[H])N3C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C3([H])[H])N=C2N1C1([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H] |
|
| InChi Key | VADOZMZXXRBXNY-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C24H27N7O/c1-29-10-12-30(13-11-29)20-8-6-19(7-9-20)27-24-26-16-18-14-17(15-25)23(32)31(22(18)28-24)21-4-2-3-5-21/h6-9,14,16,21H,2-5,10-13H2,1H3,(H,26,27,28) | |
| Chemical Name | 8-cyclopentyl-2-[4-(4-methylpiperazin-1-yl)anilino]-7-oxopyrido[2,3-d]pyrimidine-6-carbonitrile | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Cdk4/cyclin D1 (IC50 = 3.9 nM); ARK5 (IC50 = 5 nM); CDK6/cyclinD1 (IC50 = 9.82 nM); RET (IC50 = 9.2 nM); FYN (IC50 = 11 nM); FGFR1 (IC50 = 26 nM); PDGFRβ (IC50 = 26 nM); PI3K-δ (IC50 = 144 nM) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | ON123300 is a newly developed kinase inhibitor that effectively suppresses CDK4/6 and PI3K-δ, demonstrating strong efficacy against MCLs. A series of screens revealed the low molecular weight multi-kinase inhibitor ON123300, which enabled additional research into brain tumor chemotherapy. Based on biochemical assays, ON123300 was found to be a potent inhibitor of growth factor receptor tyrosine kinases, including beta-type platelet-derived growth factor receptor [PDGFRβ], as well as Ark5 and CDK4. With an IC50 = 3.4 ± 0.1 μM, ON123300 suppressed the growth of U87 glioma cells and decreased Akt phosphorylation. However, it also surprisingly activated Erk, both in a dose- and time-dependent way, which was later linked to the relief of Akt-mediated C-Raf S259 inactivation and the induction of a p70S6K-initiated PI3K negative feedback loop. | |
| Cell Assay | ON123300's cytotoxicity is assessed through a colorimetric assay based on sulforhodamine B (SRB). Glioma cell suspensions (100 mL, 2×103 cells) are seeded in 96-well plates and incubated for a whole night to allow the cells to adhere to the plate surface. Next, ON123300 is added to the cells in escalating concentrations for a duration of 72 hours. Following the course of treatment, cells are stained with 0.4% SRB and fixed with 10% (v/v) trichloroacetic acid (TCA). The wavelength at which the optical densities are determined is 570 nm. | |
| Animal Protocol |
NIH Swiss nude mice 5 and 25 mg/kg i.v. |
|
| References |
[1]. Mol Cancer Ther . 2014 May;13(5):1105-16. [2]. AAPS J . 2013 Jan;15(1):250-7. [3]. Leukemia . 2016 Jan;30(1):86-93. |
|
| Additional Infomation | Narazaciclib is an orally bioavailable inhibitor of NUAK family SNF1-like kinase 1 (AMPK-related protein kinase 5; ARK5), and the cyclin-dependent kinases 4 (CDK4) and 6 (CDK6), with potential antineoplastic activity. Upon oral administration,narazaciclib specifically binds to and inhibits ARK5, which interferes with the activation of ARK5-mediated signal transduction pathways and reduces cell proliferation in cancer cells that overexpress ARK5. In addition, ON 123300 inhibits CDK4 and 6 and prevents the phosphorylation of retinoblastoma (Rb) protein in early G1. Inhibition of Rb phosphorylation prevents CDK-mediated G1-S phase transition, which causes G1 phase cell cycle arrest, suppresses DNA synthesis and inhibits cancer cell growth. ARK5, a member of the AMP-activated protein kinase (AMPK) family, is associated with tumor growth and invasion. Overexpression of CDK4/6, which is seen in certain types of cancer, causes cell cycle deregulation. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 5 mg/mL (11.64 mM) in 0.5% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3282 mL | 11.6409 mL | 23.2818 mL | |
| 5 mM | 0.4656 mL | 2.3282 mL | 4.6564 mL | |
| 10 mM | 0.2328 mL | 1.1641 mL | 2.3282 mL |