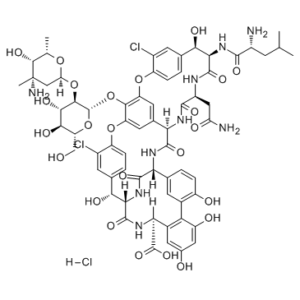
Norvancomycin (NVCM; A-51568A; N-Demethylvancomycin) hydrochloride is a macrocyclic antibiotic of the glycopeptide class that is widely used in the treatment of endocarditis, osteomyelitis, pneumonia, sepsis or soft tissue infections caused by Staphylococcus (including Methicillin-resistant strains and multidrug-resistant microbial strains). Norvancomycin is widely used in China to treat bacterial infections of Gram-positive cocci and bacilli, especially infections of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis. Norvancomycin shows pharmacological properties and antibac-terial activities similar to those of vancomycin, which exhibits significant inhibitory activity against Gram-positive cocciandbacilli, especially to methicillin-resistant Staphylococcus aureus (MRSA)andmethicillin-resistant Staphylococcusepidermidis (MRSE).Dueto its potential clinical application, norvancomycin was commercially developed by the North China Pharmaceutical Company and has been widely used in China to treat endocarditis, osteomy-elitis, and other severe infections caused by S. aureus (includingmethicillin-resistant strains) for 3 decades.
Physicochemical Properties
| Molecular Formula | C65H74CL3N9O24 | |
| Molecular Weight | 1471.7 | |
| Exact Mass | 1469.391 | |
| CAS # | 213997-73-0 | |
| Related CAS # |
|
|
| PubChem CID | 92044444 | |
| Appearance | Typically exists as solid at room temperature | |
| Hydrogen Bond Donor Count | 20 | |
| Hydrogen Bond Acceptor Count | 26 | |
| Rotatable Bond Count | 12 | |
| Heavy Atom Count | 101 | |
| Complexity | 2940 | |
| Defined Atom Stereocenter Count | 18 | |
| SMILES | ClC1C=C2C=CC=1OC1=CC3[C@H](C(N[C@H]4C(N[C@H](C(N[C@H](C(=O)O)C5C=C(C=C(C=5C5=C(C=CC4=C5)O)O)O)=O)[C@@H]2O)=O)=O)NC([C@H](CC(N)=O)NC([C@@H]([C@@H](C2C=CC(=C(C=2)Cl)OC(C=3)=C1O[C@H]1[C@@H]([C@H]([C@@H]([C@@H](CO)O1)O)O)O[C@H]1C[C@@](C)([C@@H]([C@H](C)O1)O)N)O)NC([C@@H](CC(C)C)N)=O)=O)=O.Cl |
|
| InChi Key | LLWFHCJFZGXUAT-UMXPSMRGSA-N | |
| InChi Code | InChI=1S/C65H73Cl2N9O24.ClH/c1-22(2)11-33(68)57(87)75-48-50(82)25-6-9-37(31(66)13-25)96-39-15-27-16-40(54(39)100-64-55(53(85)52(84)41(21-77)98-64)99-43-20-65(4,70)56(86)23(3)95-43)97-38-10-7-26(14-32(38)67)51(83)49-62(92)74-47(63(93)94)30-17-28(78)18-36(80)44(30)29-12-24(5-8-35(29)79)45(59(89)76-49)73-60(90)46(27)72-58(88)34(19-42(69)81)71-61(48)91;/h5-10,12-18,22-23,33-34,41,43,45-53,55-56,64,77-80,82-86H,11,19-21,68,70H2,1-4H3,(H2,69,81)(H,71,91)(H,72,88)(H,73,90)(H,74,92)(H,75,87)(H,76,89)(H,93,94);1H/t23-,33+,34-,41+,43-,45+,46+,47-,48+,49-,50+,51+,52+,53-,55+,56+,64-,65-;/m0./s1 | |
| Chemical Name | (1S,2R,18R,19R,22S,25R,28R,40S)-48-[(2S,3R,4S,5S,6R)-3-[(2S,4S,5S,6S)-4-amino-5-hydroxy-4,6-dimethyloxan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-19-[[(2R)-2-amino-4-methylpentanoyl]amino]-22-(2-amino-2-oxoethyl)-5,15-dichloro-2,18,32,35,37-pentahydroxy-20,23,26,42,44-pentaoxo-7,13-dioxa-21,24,27,41,43-pentazaoctacyclo[26.14.2.23,6.214,17.18,12.129,33.010,25.034,39]pentaconta-3,5,8(48),9,11,14,16,29(45),30,32,34(39),35,37,46,49-pentadecaene-40-carboxylic acid;hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| References | The Journal of Antibiotics,70,p158–165(2017);Journal of Chromatography B, 1072, 1, 2018, 199-204. |
Solubility Data
| Solubility (In Vitro) |
H2O : ≥ ~100 mg/mL (~67.95 mM) DMSO : ≥ ~100 mg/mL (~67.95 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (1.70 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (1.70 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (1.70 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (1.70 mM) Solubility in Formulation 5: 100 mg/mL (67.95 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.6795 mL | 3.3974 mL | 6.7949 mL | |
| 5 mM | 0.1359 mL | 0.6795 mL | 1.3590 mL | |
| 10 mM | 0.0679 mL | 0.3397 mL | 0.6795 mL |