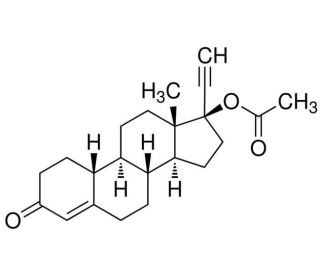
Norethindrone acetate (NSC-22844; NSC22844; NSC 22844; ENTA) is the 17-O-acetylated ester of Norethindrone (Norethisterone; sold under many trade names), which is a synthetic progestin and an orally active contraceptive for preventing pregnancy.
Physicochemical Properties
| Molecular Formula | C22H28O3 |
| Molecular Weight | 340.46 |
| Exact Mass | 340.203 |
| CAS # | 51-98-9 |
| Related CAS # | Norethindrone acetate-d8 |
| PubChem CID | 5832 |
| Appearance | White to off-white solid powder |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 454.7±45.0 °C at 760 mmHg |
| Flash Point | 197.1±28.8 °C |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.556 |
| LogP | 3.99 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 25 |
| Complexity | 695 |
| Defined Atom Stereocenter Count | 6 |
| SMILES | CC(=O)O[C@]1(CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4=CC(=O)CC[C@H]34)C)C#C |
| InChi Key | IMONTRJLAWHYGT-ZCPXKWAGSA-N |
| InChi Code | InChI=1S/C22H28O3/c1-4-22(25-14(2)23)12-10-20-19-7-5-15-13-16(24)6-8-17(15)18(19)9-11-21(20,22)3/h1,13,17-20H,5-12H2,2-3H3/t17-,18+,19+,20-,21-,22-/m0/s1 |
| Chemical Name | [(8R,9S,10R,13S,14S,17R)-17-ethynyl-13-methyl-3-oxo-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-yl] acetate |
| Synonyms | NSC-22844 NSC22844NSC 22844ENTA Norethindrone acetate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vivo | For the treatment of symptomatic endometriosis, norethindrone acetate may be a reasonably priced substitute with manageable side effects. Dysmenorrhea and non-cyclic pelvic discomfort were relieved in norethindrone acetate-treated subjects [1]. For managing pain and bleeding in all phases of endometriosis, norethindrone acetate alone is a well-tolerated and efficient treatment. Norethindrone acetate medication improved bleeding scores regardless of prior hormonal regimen, and it also improved pain scores in all patients except those who had previously been treated a GnRH agonist + add-back [2]. In laboratory animals, norethindrone acetate shows little acute toxicity, which is in line with the absence of toxicity seen in people. When norethindrone acetate was given to rodents at multiple human doses, there was no observed treatment-related mortality, hematological alteration, behavioral alteration, or organ disease [3]. When rats fed a high-carb diet were given norethindrone acetate, their plasma lipoprotein densities (<1.006) and phospholipid concentrations of cholesterol, triglycerides, and plasma lipoproteins decreased significantly and proportionately. Significantly less palmitate and glycerol uptake was observed in triglycerides given to isolated rat hepatocytes when norethindrone acetate (0.1 mM) was administered [4]. |
| References |
[1]. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998 Jan-Feb;43(1):24-7. [2]. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012 Apr;25(2):105-8. [3]. Pharmacology and toxicology of ethinyl estradiol and norethindrone acetate in experimental animals. Regul Toxicol Pharmacol. 2001 Aug;34(1):53-61. [4]. Norethindrone acetate inhibition of triglyceride synthesis and release by rat hepatocytes. Atherosclerosis. 1983 Jan;46(1):41-8. |
| Additional Infomation |
Norethisterone Acetate (Norethindrone Acetate) can cause developmental toxicity according to state or federal government labeling requirements. Norethisterone acetate is a 3-oxo Delta(4)-steroid that is norethisterone in which the hydroxy group has been converted to its acetate ester. It has a role as a synthetic oral contraceptive and a progestin. It is a 3-oxo-Delta(4) steroid, a terminal acetylenic compound and an acetate ester. It is functionally related to a norethisterone. Norethindrone Acetate is the orally bioavailable acetate salt of norethindrone, a synthetic progestin with some anabolic, estrogenic, and androgenic activities. As do all progestins, norethindrone binds to and activates nuclear progesterone receptors (PRs) in target tissues such as the pituitary and reproductive system; ligand-receptor complexes are translocated to the nucleus where they bind to progesterone response elements (PREs) located on target genes, followed by various transcriptional events and histone acetylation. Physiological effects include the inhibition of luteinizing hormone (LH) release, an increase in the endometrial luteal-phase, and alterations in endocervical mucus secretion. Acetate ester of norethindrone that is used as a long-term contraceptive (CONTRACEPTIVE AGENTS). See also: Norethindrone (has active moiety); Estradiol; Norethindrone Acetate (component of); Leuprolide acetate; norethindrone acetate (component of) ... View More ... |
Solubility Data
| Solubility (In Vitro) |
DMSO : ≥ 100 mg/mL (~293.72 mM) H2O : ~0.67 mg/mL (~1.97 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.34 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.34 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.34 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9372 mL | 14.6860 mL | 29.3720 mL | |
| 5 mM | 0.5874 mL | 2.9372 mL | 5.8744 mL | |
| 10 mM | 0.2937 mL | 1.4686 mL | 2.9372 mL |