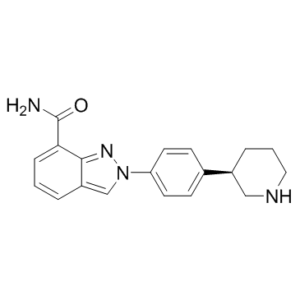
Niraparib R-enantiomer, the R-isomer of Niraparib, is a potent PARP1 [poly(ADP-Ribose) polymerase] inhibitor (IC50 = 2.4 nM) with anticancer activity. Niraparib (MK4827; MK-4827; Zejula) is a selective PARP1/2 inhibitor that the FDA approved in 2017 for adult patients in complete or partial response to platinum-based chemotherapy who have recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Physicochemical Properties
| Molecular Formula | C₁₉H₂₀N₄O | |
| Molecular Weight | 320.39 | |
| Exact Mass | 320.164 | |
| Elemental Analysis | C, 71.23; H, 6.29; N, 17.49; O, 4.99 | |
| CAS # | 1038915-58-0 | |
| Related CAS # | 1038915-64-8 (HCl); 1038915-73-9; 1613220-15-7 (tosylate hydrate); 1038915-60-4; 1476777-06-6 (Niraparib metabolite M1) | |
| PubChem CID | 24958199 | |
| Appearance | Light yellow to yellow solid powder | |
| LogP | 3.62 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 24 | |
| Complexity | 449 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | O=C(C1=CC=CC2=CN(C3=CC=C([C@@H]4CNCCC4)C=C3)N=C12)N |
|
| InChi Key | PCHKPVIQAHNQLW-AWEZNQCLSA-N | |
| InChi Code | InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m0/s1 | |
| Chemical Name | 2-[4-[(3R)-piperidin-3-yl]phenyl]indazole-7-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PARP-1 ( IC50 = 2.4 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay |
PARP-1 SPA Assay [1] Enzyme assay was conducted in buffer containing 25 mM Tris, pH 8.0, 1 mM DTT, 1 mM spermine, 50 mM KCl, 0.01% Nonidet P-40, and 1 mM MgCl2. PARP reactions contained 0.1 μCi [3H]NAD+ (200 000 DPM), 1.5 μM NAD+, 150 nM biotinylated NAD+, 1 μg/mL activated calf thymus, and 1−5 nM PARP-1. Autoreactions utilizing SPA bead-based detection were carried out in 50 μL volumes in white 96-well plates. Compounds were prepared in 11-point serial dilution in 96-well plate, 5 μL/well in 5% DMSO/H2O (10× concentrated). Reactions were initiated by adding first 35 μL of PARP-1 enzyme in buffer and incubating for 5 min at room temperature and then 10 μL of NAD+ and DNA substrate mixture. After 3 h at room temperature, these reactions were terminated by the addition of 50 μL of streptavidin-SPA beads (2.5 mg/mL in 200 mM EDTA, pH 8). After 5 min, they were counted using a TopCount microplate scintillation counter. IC50 data was determined from inhibition curves at various substrate concentrations. PARP Isoform TCA Assays [1] The enzymatic reaction was conducted in the presence of 25 mM Tris-HCl pH 8.0, 1 mM MgCl2, 50 mM KCl, 1 mM spermine, 0.01% Nonidet P-40, and 1 mM DTT. PARP reactions contained 0.1 μCi [3H]NAD (200 000 DPM), 1.5 μM NAD+, 1 μg/mL activated calf thymus, and 0.2−1 nM human PARP-1 enzyme. Assays were carried out in 50 μL volumes in white 96-well polypropylene microplate. A 96-well plate was prepared with serial dilutions over 10 points over a 0.1−50 nM concentration range 5% DMSO/H2O, 5 μL. Reactions were initiated by adding first 35 μL of PARP-1 enzyme in buffer and incubating for 5 min at room temperature, then 10 μL of NAD+ and DNA substrate mixture. After 2 h incubation at room temperature, the reaction was stopped by the addition of TCA (50 μL/well, 20% in 20 mM NaPPi solution) and incubated for 10 min over ice. The resulting precipitate was filtered on a Unifilter GF/B microplate and washed four times with 2.5% TCA. After addition of 50 μL/well of scintillation liquid the amount of radioactivity incorporated into the PAR polymers was determined using a TopCount microplate scintillation counter. IC50 data were determined from inhibition curves at various substrate concentrations. The protocols for the other PARP family members are very similar with subtle changes as described in the Supporting Information. PARylation Assay [1] HeLa cells were seeded into a 96-well Viewplate black microplate at an initial concentration of 10 000 cells/well in culture medium (100 μL of DMEM containing 10% FCS, 0.1 mg/mL penicillin−streptomycin, and 2 mM l-glutamine). The plates were incubated for 4 h at 37 °C under 5% CO2 atmosphere, and then compounds were added with serial dilutions over nine points over a 0.3−100 nM concentration range in 5% DMSO/H2O, 10 μL/well. The plate was then incubated for 18 h at 37 °C in 5% CO2, and then DNA damage was provoked by addition of 5 μL of H2O2 solution in H2O (final concentration 200 μM). As a negative control, cells untreated with H2O2 were used. The plate was kept at 37 °C for 5 min. Then the medium was gently removed by plate inversion, and the cells were fixed by addition of ice-cold MeOH (100 μL/well) and kept at −20 °C for 20 min. After removal of the fixative by plate inversion and washing 10 times with PBS (300 μL), the detection buffer (100 μL/well, containing PBS, Tween (0.05%), and BSA (1 mg/mL)) together with the primary PAR mAb (1:2000), the secondary antimouse Alexa Fluor 488 antibody (1:3000), and nuclear dye Draq5 (Alexis Bos 889001R200, 5 μM) were added. Following 3 h incubation at room temperature in the dark, removal of the solution, and washing 10 times with PBS (300 μL), the plate was read on an InCell1000. Monitoring for PAR polymer was by detection of Alexa488 at Ex. S 475_20X, Em. HQ 535_50, exposure time of 600 ms, and identification of the nuclei was by tracking Draq5 with Ex. HQ 620_60X, Em. HQ 700_75M, exposure time of 300 ms. The % PAR-positive cells was calculated by measuring the ratio between the numbers of PAR-positive nuclei over the total number of Draq5-labeled nuclei. The IC50 was determined on the basis of the residual enzyme activity in the presence of increasing PARPi concentration. In a whole cell assay, MK-4827 inhibits PARP activity with EC(50) = 4 nM and prevents the growth of cancer cells expressing mutant BRCA-1 and BRCA-2 with CC(50) in the 10-100 nM range. It also exhibits excellent inhibition of PARP 1 and 2 with IC(50) = 3.8 and 2.1 nM, respectively. |
||
| Cell Assay |
Proliferation Assay in BRCA-1 Silenced and Wild Type HeLa Cells [1] HeLa BRCA1-silenced cells were generated by transducing HeLa cells at an MOI of 100 with a lentivirus containing an H1-derived expression cassette for a shRNA against BRCA-1 and an expression cassette for GFP (GFP under the control of EF1-a promoter). Silencing of BRCA1 was more than 80% as assessed by Taqman analysis. Control BRCA wild type HeLa cells were generated by transducing them with a lentivirus expressing GFP only. Proliferation assays were conducted in 96-well black viewplates, and 300 cells/well (250 cell/well for BRCA-1 wt) in culture medium, 190 μL/well (DMEM containing 10% FCS, 0.1 mg/mL penicillin−streptomycin, and 2 mM l-glutamine), were plated and incubated for 4 h at 37 °C under 5% CO2 atmosphere. Inhibitors were then added with serial dilutions, 10 μL/well to obtain the desired final compound concentration in 0.5% DMSO. The cells were then incubated for 7 days at 37 °C in 5% CO2 after which time viability was assessed. Briefly, with CellTiter-Blue (Promega) solution prediluted 1:10 in medium, 100 μL/well was added and the cells left for 45 min at 37 °C under 5% CO2 and then a further 15 min at room temperature in the dark. The number of living cells was determined by reading the plate at fluorimeter, excitation at 550 nm and emission at 590 nm. Cell growth was expressed as the percentage growth with respect to vehicle treated cells. The concentration required to inhibit cell growth by 50% (CC50) was determined. The protocols for the other cell lines are very similar and are described in the Supporting Information. The assays for proliferation were carried out in 96-well black viewplates. 300 cells/well (250 cells/well for BRCA-1 wt) were plated in 190 μL/well of culture medium (DMEM containing 10% FCS, 0.1 mg/mL penicillin-streptomycin, and 2 mM L-glutamine), which was then incubated for four hours at 37°C in an atmosphere of 5% CO2. After that, inhibitors were added in 10-μL/well serial dilutions to achieve the target final compound concentration in 0.5% DMSO. Following a 7-day incubation period at 37°C with 5% CO2, the viability of the cells was evaluated. In summary, 100 μL/well of prediluted 1:10 CellTiter-Blue solution was added, and the cells were incubated for 45 minutes at 37°C with 5% CO2 and then for an additional 15 minutes at room temperature in the dark. By reading the plate at the fluorimeter, excitation at 550 nm, and emission at 590 nm, the number of living cells was ascertained. The percentage growth of the cells in comparison to the vehicle-treated cells was used to express cell growth. It was established what concentration (CC50) would stop cell growth 50% of the time. |
||
| Animal Protocol |
|
||
| References |
[1]. Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem. 2009 Nov 26;52(22):7170-85. |
||
| Additional Infomation | 2-{4-[(3R)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide is a 2-[4-(piperidin-3-yl)phenyl]-2H-indazole-7-carboxamide that has R-configuration. It is a potent PARP1 inhibitor with IC50 of 2.4 nM. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor and an antineoplastic agent. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1212 mL | 15.6060 mL | 31.2120 mL | |
| 5 mM | 0.6242 mL | 3.1212 mL | 6.2424 mL | |
| 10 mM | 0.3121 mL | 1.5606 mL | 3.1212 mL |