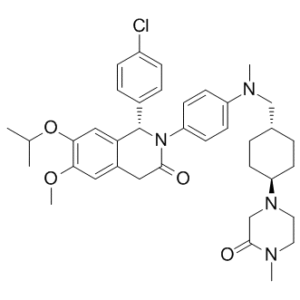
NVP-CGM097 (also called CGM-097; CGM097; NVP-CGM-097; NVP CGM097) is a novel, highly potent, orally bioavailable and selective MDM2 ( (human homolog of double minute 2) inhibitor with antitumor activity. With an IC50 of 1.7±0.1 nM, it blocks MDM2-p53. The Mdm2 protein's p53 binding site is where it binds, breaking up the interaction between the two proteins and activating the p53 pathway. CGM097, a p53/HDM2 interaction inhibitor, prevents the HDM2 protein from attaching to the p53 transcriptional activation domain when taken orally. By preventing this HDM2-p53 interaction, p53's enzymatic degradation by proteosomes is slowed down. This could lead to the restoration of p53 signaling and, consequently, the p53-mediated induction of tumor cell apoptosis.
Physicochemical Properties
| Molecular Formula | C38H47CLN4O4 |
| Molecular Weight | 659.26 |
| Exact Mass | 658.329 |
| Elemental Analysis | C, 69.23; H, 7.19; Cl, 5.38; N, 8.50; O, 9.71 |
| CAS # | 1313363-54-0 |
| Related CAS # | NVP-CGM097 (stereoisomer);2070009-54-8;NVP-CGM097 sulfate;1313367-56-4 |
| PubChem CID | 53240420 |
| Appearance | Light yellow to yellow solid powder |
| LogP | 6.524 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 47 |
| Complexity | 1040 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | ClC1C([H])=C([H])C(=C([H])C=1[H])[C@@]1([H])C2=C([H])C(=C(C([H])=C2C([H])([H])C(N1C1C([H])=C([H])C(=C([H])C=1[H])N(C([H])([H])[H])C([H])([H])C1([H])C([H])([H])C([H])([H])C([H])(C([H])([H])C1([H])[H])N1C([H])([H])C(N(C([H])([H])[H])C([H])([H])C1([H])[H])=O)=O)OC([H])([H])[H])OC([H])(C([H])([H])[H])C([H])([H])[H] |
| InChi Key | CLRSLRWKONPSRQ-CPOWQTMSSA-N |
| InChi Code | InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26?,31?,38-/m0/s1 |
| Chemical Name | (1S)-1-(4-chlorophenyl)-6-methoxy-2-[4-[methyl-[[4-(4-methyl-3-oxopiperazin-1-yl)cyclohexyl]methyl]amino]phenyl]-7-propan-2-yloxy-1,4-dihydroisoquinolin-3-one |
| Synonyms | CGM 097; CGM097; CGM-097; NVPCGM097; NVPCGM-097; NVPCGM 097; NVP CGM097; NVP CGM-097; NVP CGM 097 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | hMDM2 (IC50 = 1.7±0.1 nM) |
| ln Vitro | NVP-CGM097 binding to MDM2 is species dependent. It was shown to be selective for the p53:MDM2 interaction compared to the p53:MDM4 interaction (1176-fold selectivity) and the Ras:Raf interaction (3000-fold selectivity). Additionally, NVP-CGM097 exhibited no discernible activity against Bcl-2:Bak, Bcl-2:Bad, Mcl-1:Bak, Mcl-1:NOXA, XIAP:BIR3, and c-IAP:BIR3 protein-protein interactions. With an IC50 of 0.224 μM, NVP-CGM097 showed its capacity to block the p53:MDM2 interaction in living cells by significantly redistributing wild-type p53 into the cell nucleus. Treatment with NVP-CGM097 results in nuclear translocation of p53, which inhibits cell growth in a way that is p53-dependent[1]. |
| ln Vivo | After iv administration, the total blood clearance (CL) of NVP-CGM097 was 5 mL/min/kg for mouse, 7 mL/min/kg for rat, 3 mL/min/kg for dog, and 4 mL/min/kg for monkey. NVP-CGM097 demonstrated a consistent low total blood CL in all species (5–10% of hepatic blood flow) based on the corresponding hepatic blood flows. In rodents and monkeys, the apparent terminal half-life (t1/2) was long (6–12 h), but in dogs, it was significantly longer (20 h). The drug was effectively absorbed after oral administration, with Tmax lasting between 1 and 4.5 h in each of the tested species. In mice, rats, and dogs, the oral bioavailability (%F) was high; in monkeys, it was moderate. |
| Cell Assay | In 96-well plates, cells were seeded at the proper densities (Bon1 cells: 1500 cells/well, NCI-H727 cells: 2000 cells/well, and Got1 cells: 50000 cells/well) and grown for 24 hours in complete medium containing serum and antibiotic. The next day, the cells were incubated with various concentrations of NVP-CGM097 (0.1 nM-2500 nM), 5-fluorouracil (100 nM-100 µM), streptozotocin (1 nM-100 µM), temozolomide (1 µM-1 mM), everolimus (10 nM) or octreotide (100 nM-10 µM) in 10% FBS medium (antibiotic-free). The metabolic activity was assessed using the "Cell Titer 96 Aqueous One Solution" cell proliferation assay after 48 hours, 96 hours, 144 hours, or 216 hours. Using an ELISA plate reader, the measurement was carried out at 492 nm. |
| Animal Protocol | Rats: Female athymic rats bearing subcutaneous xenotransplants of SJSA-1 tumors (n=5-12) are treated at 5, 10, 20, or 30 mg/kg or three times a week on Monday, Wednesday, and Friday (3qw M, W, F) at 30 or 70 mg/kg po for 14 days. Plasma AUCs are determined at the end of the study. Positive numbers indicate the percentage of tumor growth inhibition (T/C); negative numbers indicate the percentage of tumor regression. |
| References |
[1]. J Med Chem . 2015 Aug 27;58(16):6348-58. [2]. Neuroendocrinology . 2018;106(1):1-19. |
| Additional Infomation | p53/HDM2 Interaction Inhibitor CGM097 is an orally bioavailable HDM2 (human homolog of double minute 2) antagonist with potential antineoplastic activity. Upon oral administration, p53/HDM2 interaction inhibitor CGM097 inhibits the binding of the HDM2 protein to the transcriptional activation domain of the tumor suppressor protein p53. By preventing this HDM2-p53 interaction, the proteasome-mediated enzymatic degradation of p53 is inhibited, which may result in the restoration of p53 signaling and, thus, the p53-mediated induction of tumor cell apoptosis. HDM2, a zinc finger nuclear phosphoprotein, is a negative regulator of the p53 pathway, often overexpressed in cancer cells and has been implicated in cancer cell proliferation and survival. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (3.79 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (3.79 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5169 mL | 7.5843 mL | 15.1685 mL | |
| 5 mM | 0.3034 mL | 1.5169 mL | 3.0337 mL | |
| 10 mM | 0.1517 mL | 0.7584 mL | 1.5169 mL |