NU1025 is a novel, potent and selective inhibitor of poly(ADP-ribose) polymerase (PARP) with potential anticancer activity. It also exhibits strong in vivo antitumor efficacy and strong antiproliferative activity against a variety of cancer cells. In L1210 cells, NU1025 has the ability to increase the cytotoxicity of a variety of anti-cancer drugs.
Physicochemical Properties
| Molecular Formula | C9H8N2O2 | |
| Molecular Weight | 176.17 | |
| Exact Mass | 176.058 | |
| CAS # | 90417-38-2 | |
| Related CAS # |
|
|
| PubChem CID | 135398517 | |
| Appearance | White solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 345.4±44.0 °C at 760 mmHg | |
| Melting Point | 253-258ºC | |
| Flash Point | 162.7±28.4 °C | |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C | |
| Index of Refraction | 1.678 | |
| LogP | 0.35 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 0 | |
| Heavy Atom Count | 13 | |
| Complexity | 262 | |
| Defined Atom Stereocenter Count | 0 | |
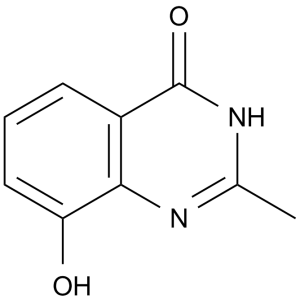
| SMILES | O=C1C2C(=C(C=CC=2)O)NC(C)=N1 |
|
| InChi Key | YJDAOHJWLUNFLX-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C9H8N2O2/c1-5-10-8-6(9(13)11-5)3-2-4-7(8)12/h2-4,12H,1H3,(H,10,11,13) | |
| Chemical Name | 8-hydroxy-2-methyl-3H-quinazolin-4-one | |
| Synonyms | NU 1025; NU1025; NU-1025 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PARP ( IC50 = 400 nM ); PARP ( Ki = 48 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | After 30 minutes on ice, cells are suspended in hypotonic buffer (9 mM HEPES, pH 7.8, 4.5% (v/v) dextran, 4.5 mM MgCl2, and 5 mM DTT) at 1.5 × 107/mL. Subsequently, 9 vol of isotonic buffer (40 mM HEPES, pH 7.8, 130 mM KCl, 4% (v/v) dextran, 2 mM EGTA, 2.3 mM MgCl2, 225 mM sucrose, and 2.5 mM DTT) is added. 2 mL of ice-cold 10% (w/v) TCA + 10% (w/v) sodium pyrophosphate is added to end the reaction, which was initiated by adding 300 µL cells to 100 µL 300 µM NAD+ containing [32P]-NAD+. The 32P-labelled ADP-ribose polymers that precipitate after 30 minutes on ice are filtered, five times cleaned with 1% (v/v) TCA and 1% (v/v) sodium pyrophosphate, dried, and counted. | ||
| Cell Assay | The indicated doses of NU1025 are applied after the cells (D54 and U251 cells) are seeded at a density of 2,500 cells/well in 96-well plates. A dose of 0.5 Gy/min of 250 kVp X-rays is applied to the medium containing adherent cells. The control group consists of untreated cells. Cell proliferation is measured using the MTT assay after an incubation period of up to five days. | ||
| Animal Protocol |
|
||
| References |
[1]. Neuroprotective effects of NU1025, a PARP inhibitor in cerebral ischemia are mediated through reduction in NAD depletion and DNA fragmentation. Life Sci. 2006 Nov 10;79(24):2293-302. [2]. Potentiation of anti-cancer agent cytotoxicity by the potent poly(ADP-ribose) polymerase inhibitors NU1025 and NU1064. Br J Cancer. 1998 Nov;78(10):1269-77. [3]. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res. 2000 Jul;6(7):2860-7. |
||
| Additional Infomation |
NU 1025 is a member of the class of quinazolines that is quinazolin-4(1H)-one substituted by a hydroxy group at position 8 and a methyl group at position 2. It has been shown to exhibit inhibitory activity against poly(ADP-ribose) polymerase. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor. It is a member of quinazolines and a member of phenols. 8-Hydroxy-2-methylquinazolin-4(3H)-one has been reported in Streptomyces with data available. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (11.81 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (11.81 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: 40% PEG 400+saline: 18mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.6763 mL | 28.3817 mL | 56.7634 mL | |
| 5 mM | 1.1353 mL | 5.6763 mL | 11.3527 mL | |
| 10 mM | 0.5676 mL | 2.8382 mL | 5.6763 mL |