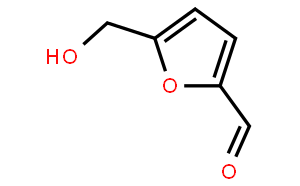
NSC-40738(5-Hydroxymethylfurfural ) is a novel and potent biochemical for treatment of sickle cell anemia. which is a stressor, prevents fermentation and yeast growth. It is sourced from Cornus officinalis.
Physicochemical Properties
| Molecular Formula | C6H6O3 |
| Molecular Weight | 126.11 |
| Exact Mass | 126.031 |
| Elemental Analysis | C, 57.14; H, 4.80; O, 38.06 |
| CAS # | 67-47-0 |
| Related CAS # | 5-Hydroxymethylfurfural-13C6;1219193-98-2 |
| PubChem CID | 237332 |
| Appearance | Light yellow to light brown solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 291.5±30.0 °C at 760 mmHg |
| Melting Point | 28-34 °C(lit.) |
| Flash Point | 79.4±0.0 °C |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.563 |
| LogP | -0.45 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 9 |
| Complexity | 103 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O1C(C([H])=O)=C([H])C([H])=C1C([H])([H])O[H] |
| InChi Key | NOEGNKMFWQHSLB-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C6H6O3/c7-3-5-1-2-6(4-8)9-5/h1-3,8H,4H2 |
| Chemical Name | 5-(hydroxymethyl)furan-2-carbaldehyde |
| Synonyms | NSC-40738; BAX-555; 5-HMF; AES-103; NSC40738; BAX555; 5HMF; AES103; NSC 40738; BAX 555; 5 HMF; AES 103;5-HMF-AesRx; 5-hydroxymethyl furfural |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Yeast |
| ln Vitro | It is discovered that in Saccharomyces cerevisiae, furfural and HMF lead to the formation of cytoplasmic mRNP granules and the reduction of bulk translation activity. SG formation and translation initiation are notably suppressed when furfural and HMF are combined. Cytoplasmic mRNP granules can be induced by furfural and HMF. HMF also gradually lowers the polysome fraction while simultaneously raising the 80S monosome fraction[1]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion .../This study/ determined the /5-Hydroxymethylfurfural/ (HMF) content in Norwegian food items and estimated the dietary intake of HMF in 53 volunteers by means of 24 hr dietary recall. The estimated intakes of HMF were correlated with urinary excretion of /5-hydroxymethyl-2-furoic acid/ (HMFA). Coffee, prunes, dark beer, canned peaches and raisins had the highest levels of HMF. The 95th percentile of the estimated daily dietary intake of HMF and the 24hr urinary excretion of HMFA were 27.6 and 28.6 mg, respectively. Coffee, dried fruit, honey and alcohol were identified as independent determinants of urinary HMFA excretion. Most participants had lower estimated HMF intake than the amount of HMFA excreted in urine. In spite of this there was a significant correlation (r=0.57, P<0.001) between the estimated HMF intake and urinary HMFA... ....In a small human study with seven healthy volunteers the urine excretion of unmetabolised 5-hydroxymethylfurfural was investigated. After uptake of 20 g of plum jam containing 24 mg of 5-hydroxymethylfurfural, 163 ug (mean) were excreted within 6 hr, an equivalent of 0.75% of the ingested 5-hydroxymethylfurfural. Metabolism / Metabolites 5-Hydroxymethylfurfural (HMF) is formed in carbohydrate-rich food during acid-catalysed dehydration and in the Maillard reaction from reducing sugars. HMF is found in mg quantities per kg in various foods. HMF is mainly metabolised to 5-hydroxymethyl-2-furoic acid (HMFA), but unknown quantities of the mutagenic 5-sulphoxymethylfurfural (SMF) may also be formed, making HMF potentially hazardous to humans... 5-Hydroxymethylfurfural (HMF) is formed when sugars are acidified or heated. It is present at high levels in numerous foods. HMF is inactive in standard genotoxicity tests, but can be metabolized to a chemically reactive intermediate, 5-sulfooxymethylfurfural (SMF), which is mutagenic and carcinogenic. ...direct parental administration of SMF to mice leads to abundant acute necrosis and proteinaceous casts in the proximal tubules as the dominating toxicological effect. Since proximal tubule cells actively mediate the excretion of many organic anions, we hypothesized that transporter-mediated uptake of SMF into the cells could be the reason for this selective organotoxicity. To test this hypothesis...human embryonic kidney (HEK293) cells stably expressing human (h) OAT1 or OAT3 /were used/. SMF was a competitive inhibitor of p-aminohippurate uptake by hOAT1 and estrone sulfate uptake by hOAT3 with K(i) values of 225 uM and 1.5mM, respectively. Moreover, the initial rates of SMF uptake were 5.2- and 3.1-fold higher in cells expressing hOAT1 and hOAT3, respectively, than in control HEK293 cells. Likewise, the sensitivity of hOAT1- and hOAT3-expressing cells to SMF cytotoxicity was significantly higher than that of control cells, and was reduced by addition of probenecid, an inhibitor of OATs. Taken together, these results indicate that OAT1 and OAT3 mediate the uptake of SMF into proximal tubule cells and thereby may be involved in SMF-induced nephrotoxicity. 5-Hydroxymethylfurfural (HMF), formed by acid-catalyzed dehydration and in the Maillard reaction from reducing sugars, is found at high levels in numerous foods. It was shown to initiate colon aberrant crypt foci in rats and skin papillomas and hepatocellular adenomas in mice. HMF is inactive in in vitro genotoxicity tests using standard activating systems but is activated to a mutagen by sulfotransferases. The product, 5-sulfoxymethylfurfural (SMF), is a stronger carcinogen than HMF. SMF has not been detected in the biotransfomation experiments conducted on HMF in humans and animals in vivo up to date. /This study/ report pharmacokinetic properties of HMF and SMF in FVB/N mice. Sensitive assays for the quantification of HMF and SMF by LC-MS/MS multiple reaction monitoring were devised. SMF, intravenously injected (4.4 umol/kg body mass), showed first-order elimination kinetics in blood plasma (t(1/2) = 7.9 min). HMF, injected intravenously (793 umol/kg body mass), demonstrated biphasic kinetics in plasma (t(1/2) = 1.7 and 28 min for the initial and terminal elimination phases, respectively); the volume of distribution of the central compartment corresponded approximately to the total body water. The maximum SMF plasma level was observed at the first sampling time, 2.5 min after HMF administration. On the basis of these kinetic data, it was estimated that between 452 and 551 ppm of the initial HMF dose was converted to SMF and reached the circulation. It is likely that additional SMF reacted with cellular structures at the site of generation and thus is ignored in this balance... 5-Hydroxymethyl-2-furaldehyde (HMF), is a major product of sugar degradation found in food and solutions used in parenteral nutrition. Labeled [(14)C]HMF was synthesized by dehydration of [(14C)]fructose on ion-exchange resin and administered per os (po) and intravenously (iv) to rats. Metabolic balance of radioactivity demonstrated that HMF or its metabolites are rapidly eliminated in the urine with a recovery of 95-100% after 24 hr. Literature reported, in some cases, 50% retention in the body. HMF was completely converted to two metabolites, which have been identified by nuclear magnetic resonance (NMR) and mass spectroscopy (MS) as 5-hydroxymethyl-2-furoic acid and N-(5-hydroxymethyl-2-furoyl)glycine. Administration of high doses of HMF showed a similar rapid elimination, but a proportional reduction of the amount of the glycine conjugate produced. Whole-animal-body autoradiography confirm that shortly after administration radioactive material was present in the liver but was mostly in the kidney and the bladder. The only significant difference between po and iv administration was the presence of a higher level of radioactive material in the brain of iv-treated rats. For more Metabolism/Metabolites (Complete) data for 5-Hydroxymethyl-2-furfuraldehyde (8 total), please visit the HSDB record page. 5-hydroxymethylfurfural has known human metabolites that include 5-Sulfooxymethylfurfural. |
| Toxicity/Toxicokinetics |
Interactions Our previous study reported that co-administration of honey significantly increased the serum levels of glycyrrhetic acid (GA) after oral administration of glycyrrhizin (GZ) in rabbits. The components of honey are sucrose, glucose, fructose and 5-hydroxymethyl-furaldehyde (HMF). To clarify the causative component(s) in honey that altered the metabolic pharmacokinetics of GZ, rabbits were given GZ (150 mg /per/ kg) with and without glucose (5 g /per/ rabbit), fructose (5 g /per/ rabbit) and HMF (1 mg /per/ kg), respectively, in crossover designs. An HPLC method was used to determine concentrations of GZ and GA in serum as well as GA and 3-dehydroglycyrrhetic acid (3-dehydroGA) in feces suspension. A noncompartment model was used to calculate the pharmacokinetic parameters and analysis of variance was used for statistical comparison. Our results indicated that the area under curve (AUC) of GA was significantly increased by 29% when HMF was coadministered, whereas the pharmacokinetics of GZ and GA were not significantly altered by coadministration of glucose or fructose. An in-vitro study, using feces to incubate GZ and GA individually, indicated that HMF significantly inhibited the oxidation of GA to 3-dehydroGA and this may explain the enhanced GA absorption in-vivo. It was concluded that HMF is the causative component in honey that affects the presystemic metabolism and pharmacokinetics of GZ in-vivo. Chemical analysis of several brands of peritoneal dialysis fluids (PD fluids) has revealed the presence of 2-furaldehyde, 5-HMF (5-hydroxymethylfuraldehyde), acetaldehyde, formaldehyde, glyoxal, and methylglyoxal. The aim of this study was to investigate if the in vitro side effects caused by glucose degradation products, mainly formed during heat sterilization, are due to any of these recently identified aldehydes. Cell growth media or sterile filtered PD fluids were spiked with different concentrations of thealdehydes. In vitro side effects were determined as the inhibition of cell growth of cultured mouse fibroblasts or stimulated superoxide radical release from human peritoneal cells. Our results demonstrate that the occurrences of 2-furaldehyde, 5-HMF, acetaldehyde, formaldehyde, glyoxal, or methylglyoxal in heat-sterilized PD fluids are probably not the direct cause of in vitro side effects. In order to induce the same magnitude of cell growth inhibition as the heat-sterilized PD fluids, the concentrations of 2-furaldehyde, glyoxal, and 5-HMF had to be 50 to 350 times higher than those quantified in the PD fluids. The concentrations of acetaldehyde, formaldehyde, and methylglyoxal observed in the heat-sterilized PD fluids were closer to the cytotoxic concentrations although still 3 to 7 times lower. Since none of these aldehydes caused in vitro toxicity at the tested concentrations, the toxicity found in PD fluids is likely to be due to another glucose degradation product, not yet identified. However, it is possible that these aldehydes may still have adverse effects for patients on peritoneal dialysis. The effect of water activity (aw 0.98, 0.84 and 0.60) and reaction temperature (100, 120, 140 and 160 degrees C) on the mutagenic activity of the Maillard reaction products in heated ribose-lysine and glucose-lysine model systems, was investigated. In the ribose-lysine system, heated at 100 °C, the mutagenic activity of the mixture increased as the water activity was lowered. On the contrary, no dependence between mutagenic activity and water activity was observed in the glucose-lysine system. At higher temperatures, in both systems, the presence in the browned mixtures of an antibacterial activity interfering with the bacterial mutagenicity assay was observed. Under all the conditions tested, the ribose-lysine system turned out to be the most reactive by producing higher levels of mutagens. Furthermore, in this system, the antimicrobial interference was more easily detectable. In the model systems used, the browning reaction mixtures were analysed for their absorption spectrum between 200-460 nm, and for the accumulation of furfurals. The results obtained showed that, at temperatures between 120 and 140 degrees C there is a correlation among reaction temperature, absorbance at 420 and around 280 nm, mutagenic activity of the mixture and the level of furfurals. Changes in the levels of furfurals can be related to changes in mutagenicity of the browned mixtures. Dietary casein cooked at 180 °C promotes the growth of aberrant crypt foci and colon cancer in rats initiated with azozymethane. We speculated that promotion was due to a product that could be extracted by a solvent, such as 5-hydroxymethyl-2-furaldehyde (HMF), with tumor promoting activity or the carcinogenic heterocyclic aromatic amines (HAA). This hypothesis was tested by extracting cooked casein with solvents and water The extracts were then 1) assayed by high-performance liquid chromatography for HMF and HAA, 2) measured for mutagenicity on a frame-shift-sensitive strain of Salmonella typhimurium, 3) fed for 100 days to azoxymethane-initiated rats to test the promoting effect on aberrant crypt foci. Data show that 1) no HMF or HAA was detected in cooked casein, 2) no mutagenicity was detected on strain TA98, with or without metabolic activation, and 3) promotion was not associated with the extracts but with the cooked casein residue... For more Interactions (Complete) data for 5-Hydroxymethyl-2-furfuraldehyde (6 total), please visit the HSDB record page. |
| References |
[1]. Biomass conversion inhibitors furfural and 5-hydroxymethylfurfural induce formation of messenger RNP granules and attenuate translation activity in Saccharomyces cerevisiae. Appl Environ Microbiol. 2013 Mar;79(5):1661-7. |
| Additional Infomation |
Drug Warnings When glucose is present in a medical fluid, the heat applied during sterilization leads to degradation. The glucose degradation products (GDPs) give rise to bioincompatible reactions in peritoneal dialysis patients. The extent of the degradation depends on a number of factors, such as heating time, temperature, pH, glucose concentration, and catalyzing substances. In the present work investigated the influence of pH and concentration in order to determine how to decrease the amounts of GDPs produced. Glucose solutions (1%-60% glucose; pH 1-8) were heat sterilized at 121 °C. Ultraviolet (UV) absorption, aldehydes, pH, and inhibition of cell growth (ICG) were used as measures of degradation. Glucose degradation was minimum at an initial pH (prior to sterilization) of around 3.5 and at a high concentration of glucose. There was considerable development of acid degradation products during the sterilization process when the initial pH was high. Two different patterns of development of UV-absorbing degradation products were seen: one below pH 3.5, dominated by the formation of 5-hydroxy-methyl-2-furaldehyde (5-HMF); and one above, dominated by degradation products absorbing at 228 nm. 3-Deoxyglucosone (3-DG) concentration and the portion of 228 nm UV absorbance not caused by 5-HMF were found to relate to the in vitro bioincompatibility measured as ICG; there was no relation between 5-HMF or absorbance at 284 nm and bioincompatibility. In order to minimize the development of bioincompatible GDPs in peritoneal dialysis fluids during heat sterilization, pH should be kept around 3.2 and the concentration of glucose should be high. 5-HMF and 284 nm UV absorbance are not reliable as quality measures. 3-DG and the portion of UV absorbance at 228 nm caused by degradation products other than 5-HMF seem to be reliable indicators of bioincompatibility. The levels of the degradation product, 5-hydroxymethylfurfural (I), in Dextrose Injection USP were determined by UV spectrophotometry. A freshly prepared solution of 50% dextrose had a I level of 0.10 ug/mL. Within 24 hr of manufacturing, the level was 0.72 ug/mL. The level of I in 50% dextrose injection, after storage for 4 months at 70 F, was 5.80 ug/mL. Data are also reported for 10% fructose injection. It is concluded that limits for I levels in commercially available solutions can be established. It is recommended that a quantitative procedure for determining this impurity be included in quality control testing of dextrose injection. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~25 mg/mL ( ~198.23 mM ) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (19.82 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (19.82 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (19.82 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (19.82 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.9296 mL | 39.6479 mL | 79.2959 mL | |
| 5 mM | 1.5859 mL | 7.9296 mL | 15.8592 mL | |
| 10 mM | 0.7930 mL | 3.9648 mL | 7.9296 mL |