Physicochemical Properties
| Molecular Formula | C15H11NO4 |
| Molecular Weight | 269.25 |
| Exact Mass | 269.068 |
| CAS # | 2910757-30-9 |
| PubChem CID | 163409047 |
| Appearance | Typically exists as solid at room temperature |
| LogP | 2.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 20 |
| Complexity | 356 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | IWQSFILCQBTRKR-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C15H11NO4/c17-11-6-9-7-14(20-13(9)8-12(11)18)15(19)16-10-4-2-1-3-5-10/h1-8,17-18H,(H,16,19) |
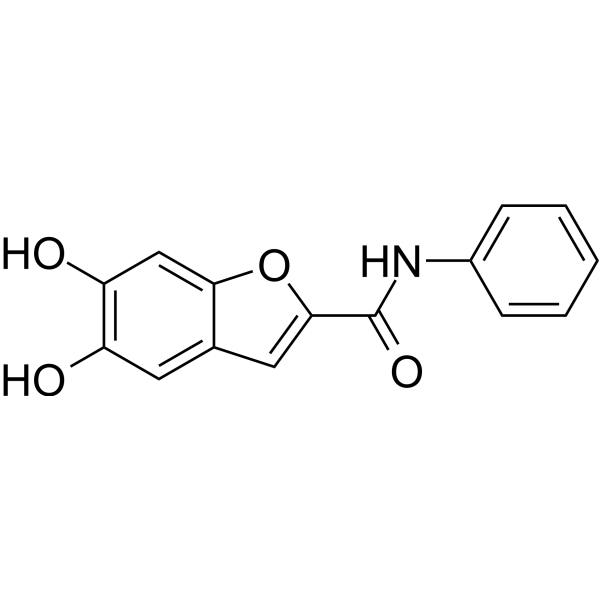
| Chemical Name | 5,6-dihydroxy-N-phenyl-1-benzofuran-2-carboxamide |
| Synonyms | NS2B/NS3-IN-4; CHEMBL4865575; 2910757-30-9; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | DENV2 NS2B/NS3 protease (IC50 = 0.69 µM); ZIKV NS2B/NS3 protease (IC50 = 1.04 µM) |
| ln Vitro | In this work, researchers elucidate new structure-activity relationships of benzo[d]thiazole-based allosteric NS2B/NS3 inhibitors. They developed a new series of Y-shaped inhibitors, which, with its larger hydrophobic contact surface, should bind to previously unaddressed regions of the allosteric NS2B/NS3 binding pocket. By scaffold-hopping, researchers varied the benzo[d]thiazole core and identified benzofuran as a new lead scaffold shifting the selectivity of initially ZIKV-targeting inhibitors to higher activities towards the DENV protease. In addition, researchers were able to increase the ligand efficiency from 0.27 to 0.41 by subsequent inhibitor truncation and identified N-(5,6-dihydroxybenzo[d]thiazol-2-yl)-4-iodobenzamide as a novel sub-micromolar NS2B/NS3 inhibitor. Utilizing cell-based assays, researchers could prove the antiviral activity in cellulo. Overall, researchers report new series of sub-micromolar allosteric DENV and ZIKV inhibitors with good efficacy profile in terms of cytotoxicity and protease inhibition selectivity. [1] |
| Enzyme Assay |
Fluorometric assays [1] The determination of the inhibitory activity of the compounds against the proteases was performed with an assay based on the fluorogenic substrates or FRET-based substrates. The inhibitors and the substrate were prepared as stock solutions in DMSO. The fluorescence was measured in white flat-bottom 96-well microtiter plates using a Tecan Infinite F2000 PRO plate reader. Measurements were performed in at least three independent experiments. In each well a total volume of 200 µL was used, consisting of 180 µL buffer, 5 µL enzyme solution, 10 µL inhibitor in DMSO or pure DMSO as control, and 5 µL solution of the corresponding substrate. Initial screenings were performed at inhibitor concentrations of 20 µM. IC50 values were determined with dilution series between 0.01 µM and 100 µM. The fluorescence was measured every 30 s for 10 min at 25 °C with the corresponding excitation and emission wavelengths. IC50 values were calculated with GraFit by fitting the remaining enzymatic activity to the four-parameter IC50 equation, with Y [ΔF/min] as the substrate hydrolysis rate, Ymax as the maximum value of the dose–response curve, measured at inhibitor concentrations of [I] = 0 µM, Ymin as the minimum value, obtained at high inhibitor concentrations, and s as the Hill coefficient. |
| Cell Assay |
Replication analysis and luciferase assays [1] Quantification of luciferase activity was used to determine ZIKV and DENV2 RNA replication as described previously.68, 69 In brief, single-cell suspensions of Huh7 cells were prepared by trypsinization and washed once with phosphate-buffered saline. Cells were resuspended at a concentration of 1 × 10~7 cells per mL in Cytomix containing 2 mM ATP and 5 mM glutathione. 10 μg of in vitro transcribed RNA was mixed with 400 μL of the cell suspension (4x10~6 cells) and transfected by electroporation using a Gene Pulser system in a cuvette with a gap width of 0.4 cm at 975 μF and 270 V.82 Cells were resuspended in 15 mL culture medium. Cells were seeded in duplicate wells in a 12-well plate: 500 µL for the time points 4 h and 24 h and 1 mL for the time points 48 h, 72 h, and 96 h. 4 h after electroporation, cells were treated with 10 µM compound or an equivalent volume of DMSO, as vehicle control, in culture medium supplemented with 15 mM HEPES. Compound- or DMSO-containing culture medium was replenished after 48 h. Cells were lysed at 4, 24, 48, 72, and 96 h after electroporation by addition of 250 µL luciferase lysis buffer (0.1% (v/v) TritonX-100, 25 mM glycylglycine, 15 mM MgSO4, 15 mM K3PO4 pH 7.8, 4 mM EGTA, 10% (v/v) glycerol, and 1 mM DTT). For detection of Renilla luciferase activity, 100 μL lysate was mixed with 200 µL luciferase assay buffer (25 mM glycylglycine, 15 mM MgSO4, 15 mM K3PO4 pH 7.8, and 4 mM EGTA) supplemented with 14 or 28 nM coelanterazine (P.J.K). For detection of firefly luciferase activity, 100 μL lysate was mixed with 350 μL luciferase assay buffer freshly supplemented with 1 mM DTT and 2 mM ATP, and d-luciferin substrate (200 µM d-Luciferin, P.J.K.) in 25 mM glycylglycine. Cell viability assay [1] To determine the impact of compound treatment on cell viability, Huh7 cells were seeded at a density of 4x10~3 cells per well in white-walled 96-well plates and one day after seeding cells were treated with 1.25, 2.5, 5, 10, 20, and 40 µM compound or equivalent volumes of DMSO for 96 h. Cell viability was measured using the CellTiter-Glo Luminescent Cell Viability Assay following manufacturer instructions. Cell viability was determined by measurement with a plate luminometer and values normalized to untreated cells. |
| References |
[1]. SAR of novel benzothiazoles targeting an allosteric pocket of DENV and ZIKV NS2B/NS3 proteases. Bioorg Med Chem. 2021 Oct 1;47:116392. |
| Additional Infomation |
In recent years, dengue virus (DENV) and Zika virus (ZIKV), both mosquito-borne members of the Flaviviridae family, have emerged as intercontinental health issues since their vectors have spread from their tropical origins to temperate climate zones due to climate change and increasing globalization. DENV and ZIKV are positive-sense, single-stranded RNA viruses, whose genomes consist of three structural (capsid, membrane precursor, envelope) and seven non-structural (NS) proteins, all of which are initially expressed as a single precursor polyprotein. For virus maturation, the polyprotein processing is accomplished by host proteases and the viral NS2B/NS3 protease complex, whose inhibitors have been shown to be effective antiviral agents with loss of viral pathogenicity.[1] In this work, we have optimized allosteric inhibitors for the DENV2 and ZIKV NS2B/NS3 proteases. Hereby, we found new lead structures with good inhibitory properties. By exchanging the individual moieties of the lead structures 1a,b, different series of inhibitors were synthesized and investigated regarding their inhibitory effects on the NS2B/NS3 proteases of DENV2 and ZIKV. Replacing the amino acid linker with (R/S)-alanine (8a,b), isoleucine (8c), tert-leucine (8d), phenylalanine (8e), tyrosine (12a), and aspartic acid (12b) did not significantly improve the inhibitory effect. Nevertheless, the ZIKV protease was inhibited by the compounds 8a–f and 12a in the low micromolar range (IC50 = 2.13–6.48 µM). In the series of newly designed Y-shaped inhibitors (Table 2), 2,2-diphenylacetic acid derivate 23b was the most promising compound with a sub-micromolar IC50 for ZIKV (0.95 µM). Y-shaped compounds 23b and 20b as well as AA-based compounds with hydrophobic side chains 8c–f demonstrated with IC50 values in the low micromolar range that addressing both binding subsites with Y-shaped inhibitors is a promising strategy for further improvement. By truncating the inhibitor scaffold (Table 3), we were able for the first time to achieve inhibition in the low micromolar range for the DENV2 protease and simultaneously increased the ligand efficacy to obtain new starting points for further DENV2 drug discovery. The iodine-substituted inhibitor 25b was the most promising compound of this series with an IC50 of 4.38 µM for DENV2 and a sub-micromolar IC50 of 0.67 µM for ZIKV, respectively. By exchanging the benzo[d]thiazole core heterocycle, we could show that other heteroaromatic systems can serve as scaffolds besides benzo[d]thiazoles. In this SAR series, the benzofuran derivate 34e showed the best inhibitory properties (IC50(DENV2) = 0.69 µM, IC50(ZIKV) = 1.04 µM). In contrast to the benzo[d]thiazole-based compounds, 34e inhibits both the ZIKV and the DENV2 NS2B/NS3 proteases in the same order of magnitude. Screening of selected compounds against various serine and cysteine proteases demonstrated excellent off-target selectivity of our inhibitors. The most potent inhibitors and their respective methoxy prodrugs were administered in a cell-based assay for their antiviral potential to interfere with DENV2 and ZIKV replication. The compounds 22b, 23b, and 25b highlighted their antiviral potential by significantly attenuating DENV2 replication, whereas 25b and 34e showed reduced ZIKV replication. In summary, we identified two promising compounds that resemble suitable starting points for upcoming drug development. First, N-(5,6-dihydroxybenzo[d]thiazol-2-yl)-4-iodobenzamide (25b) and second, 5,6-dihydroxy-N-phenylbenzofuran-2-carboxamide (34e), both of them containing only 20 and 21 heavy atoms, respectively, and with ligand efficiencies above 0.4 are good starting points for further NS2B/NS3 inhibitor development.[1] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7140 mL | 18.5701 mL | 37.1402 mL | |
| 5 mM | 0.7428 mL | 3.7140 mL | 7.4280 mL | |
| 10 mM | 0.3714 mL | 1.8570 mL | 3.7140 mL |