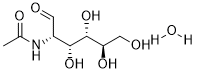
N-Acetyl-d-mannosamine (also known as N-Acetyl-D-mannosamine; ManNAc; DEX M-74) is a compound that has the potential for the treatment of neural disorders such as hereditary inclusion body myopathyan. It is also an essential precursor of N-acetylneuraminic acid (NeuAc), the specific monomer of bacterial capsular polysialic acid (PA). N-Acetyl-D-mannosamine (ManNAc) can be metabolized by GNE and GlcNAc 2-epimerase (Renin binding protein, RnBP), into ManNAc-6-phosphate and GlcNAc, respectively. N-Acetyl-d-mannosamine (ManNAc) and its derivatives activates hypocretin (HCRT) gene expression in the orexin neurons, providing a potential model for the testing of a therapy for neural disorders.
Physicochemical Properties
| Molecular Formula | C8H15NO6 |
| Molecular Weight | 221.2078 |
| Exact Mass | 221.09 |
| Elemental Analysis | C, 40.17; H, 7.16; N, 5.86; O, 46.82 |
| CAS # | 3615-17-6 |
| Related CAS # | Cyclic N-Acetyl-D-mannosamine;7772-94-3;N-Acetyl-D-mannosamine-13C;N-Acetyl-D-mannosamine-13C-1;N-Acetyl-D-mannosamine-15N |
| Appearance | White to off-white solid powder |
| Density | 1.423g/cm3 |
| Boiling Point | 636.4ºC at 760mmHg |
| Melting Point | 205ºC |
| Flash Point | 338.7ºC |
| Index of Refraction | 1.575 |
| SMILES | CC(N[C@@H]([C@@H](O)[C@H](O)[C@H](O)CO)C=O)=O |
| InChi Key | KVWIBLJBIFTKIZ-XNJRRJNCSA-N |
| InChi Code | InChI=1S/C8H15NO6.H2O/c1-4(12)9-5(2-10)7(14)8(15)6(13)3-11;/h2,5-8,11,13-15H,3H2,1H3,(H,9,12);1H2/t5-,6-,7-,8-;/m1./s1 |
| Chemical Name | N-((2S,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)acetamide hydrate |
| Synonyms | N-Acetyl-D-mannosamine (ManNAc); DEX-M-74; DEX M-74; DEXM74; DEX M 74; ManNAc; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Human Endogenous Metabolite |
| ln Vitro |
N-Acetyl-D-mannosamine (ManNAc) and N-acetyl-D-glucosamine (GlcNAc) are the essential precursors of N-acetylneuraminic acid (NeuAc), the specific monomer of polysialic acid (PA), a bacterial pathogenic determinant. Escherichia coli K1 uses both amino sugars as carbon sources and uptake takes place through the mannose phosphotransferase system transporter, a phosphoenolpyruvate-dependent phosphotransferase system that shows a broad range of specificity. Glucose, mannose, fructose, and glucosamine strongly inhibited the transport of these amino-acetylated sugars and GlcNAc and ManNAc strongly affected ManNAc and GlcNAc uptake, respectively. The ManNAc and the GlcNAc phosphorylation that occurs during uptake affected NeuAc synthesis in vitro. These findings account for the low in vivo PA production observed when E. coli K1 uses ManNAc or GlcNAc as a carbon source for growth. [1] Orexin neurons regulate critical brain activities for controlling sleep, eating, emotions, and metabolism, and impaired orexin neuron function results in several neurologic disorders. Therefore, restoring normal orexin function and understanding the mechanisms of loss or impairment of orexin neurons represent important goals. As a step toward that end, we generated human orexin neurons from induced pluripotent stem cells (hiPSCs) by treatment with N-acetyl-d-mannosamine (ManNAc) and its derivatives. The generation of orexin neurons was associated with DNA hypomethylation, histone H3/H4 hyperacetylation, and hypo-O-GlcNAcylation on the HCRT gene locus, and, thereby, the treatment of inhibitors of SIRT1 and OGT were effective at inducing orexin neurons from hiPSCs. The prolonged exposure of orexin neurons to high glucose in culture caused irreversible silencing of the HCRT gene, which was characterized by H3/H4 hypoacetylation and hyper-O-GlcNAcylation. The DNA hypomethylation status, once established in orexin neurogenesis, was maintained in the HCRT-silenced orexin neurons, indicating that histone modifications, but not DNA methylation, were responsible for the HCRT silencing. Thus, the epigenetic status of the HCRT gene is unique to the hyperglycemia-induced silencing. Intriguingly, treatment of ManNAc and its derivatives reactivated HCRT gene expression, while inhibitors SIRT1 and the OGT did not. The present study revealed that the HCRT gene was silenced by the hyperglycemia condition, and ManNAc and its derivatives were useful for restoring the orexin neurons.[2] |
| ln Vivo |
N-acetyl-D-mannosamine (administered orally in drinking water at 1% once daily for 16 weeks) prevents obesity-induced hypertension in a mouse model of high-fat diet-induced hypertension by increasing sialylation of IgG glycans [3]. N-acetyl-D-mannosamine (administered orally in drinking water at 0.5% for 8 weeks, ad libitum) improves synaptic transmission and long-term potentiation (LTP) in 6- and 14-month-old SAMR1 mice [4]. N-acetyl-D-mannosamine (administered orally in drinking water at 0.5% for 8 weeks, ad libitum) improves synaptic transmission and long-term potentiation (LTP) in 6- and 14-month-old SAMR1 mice [4]. |
| Animal Protocol |
Animal model: C57BL/6 mouse model of high-fat diet-induced hypertension [2] Dosage: 1% in drinking water Administration: Oral gavage (p.o.), once a day for 16 weeks Results: Inhibited high-fat diet-induced weight gain and increased sialylation of IgG glycans. Animal model: Knock-in C57BL/6J mouse model of GNE myopathy (Gne p.M712T) Dosage: 1 or 2 g/kg Administration: Administration via drinking water (4-6 mL per day), once a day for 12 weeks Results: Significant reduction in proteinuria (2 g/kg) after only one week of treatment |
| References |
[1]. Transport of N-acetyl-D-mannosamine and N-acetyl-D-glucosamine in Escherichia coli K1: effect on capsular polysialic acid production. FEBS Lett. 2002 Jan 30;511(1-3):97-101. [2]. Reactivation of hyperglycemia-induced hypocretin (HCRT) gene silencing by N-acetyl-d-mannosamine in the orexin neurons derived from human iPS cells. Epigenetics. 2017 Sep;12(9):764-778. [3]. Supplementation With the Sialic Acid Precursor N-Acetyl-D-Mannosamine Breaks the Link Between Obesity and Hypertension. Circulation. 2019 Dec 10;140(24):2005-2018. [4]. Oral monosaccharide therapies to reverse renal and muscle hyposialylation in a mouse model of GNE myopathy. Mol Genet Metab. 2012 Dec;107(4):748-55. [5]. Chronic administration of N-acetyl-D-mannosamine improves age-associated impairment of long-term potentiation in the senescence-accelerated mouse. Neurosci Lett. 2015 Jun 26;598:41-6. |
| Additional Infomation |
Background: Obesity-related hypertension is a common disorder, and attempts to combat the underlying obesity are often unsuccessful. We previously revealed that mice globally deficient in the inhibitory immunoglobulin G (IgG) receptor FcγRIIB are protected from obesity-induced hypertension. However, how FcγRIIB participates is unknown. Studies were designed to determine if alterations in IgG contribute to the pathogenesis of obesity-induced hypertension.
Methods: Involvement of IgG was studied using IgG μ heavy chain-null mice deficient in mature B cells and by IgG transfer. Participation of FcγRIIB was interrogated in mice with global or endothelial cell-specific deletion of the receptor. Obesity was induced by high-fat diet (HFD), and blood pressure (BP) was measured by radiotelemetry or tail cuff. The relative sialylation of the Fc glycan on mouse IgG, which influences IgG activation of Fc receptors, was evaluated by Sambucus nigra lectin blotting. Effects of IgG on endothelial NO synthase were assessed in human aortic endothelial cells. IgG Fc glycan sialylation was interrogated in 3442 human participants by mass spectrometry, and the relationship between sialylation and BP was evaluated. Effects of normalizing IgG sialylation were determined in HFD-fed mice administered the sialic acid precursor N-acetyl-D-mannosamine (ManNAc).
Results: Mice deficient in B cells were protected from obesity-induced hypertension. Compared with IgG from control chow-fed mice, IgG from HFD-fed mice was hyposialylated, and it raised BP when transferred to recipients lacking IgG; the hypertensive response was absent if recipients were FcγRIIB-deficient. Neuraminidase-treated IgG lacking the Fc glycan terminal sialic acid also raised BP. In cultured endothelial cells, via FcγRIIB, IgG from HFD-fed mice and neuraminidase-treated IgG inhibited vascular endothelial growth factor activation of endothelial NO synthase by altering endothelial NO synthase phosphorylation. In humans, obesity was associated with lower IgG sialylation, and systolic BP was inversely related to IgG sialylation. Mice deficient in FcγRIIB in endothelium were protected from obesity-induced hypertension. Furthermore, in HFD-fed mice, ManNAc normalized IgG sialylation and prevented obesity-induced hypertension.
Conclusions: Hyposialylated IgG and FcγRIIB in endothelium are critically involved in obesity-induced hypertension in mice, and supportive evidence was obtained in humans. Interventions targeting these mechanisms, such as ManNAc supplementation, may provide novel means to break the link between obesity and hypertension. 3] N-Acetyl-D-mannosamine (ManNAc), a precursor of a sialic acid, is recently reported to improve the cognitive function in aged animals. However, the effect of chronic administration of ManNAc on impaired synaptic transmission and plasticity with age still remain unknown. In this study, we electrophysiologically determined the effect of chronic administration of ManNAc on deteriorated synaptic transmission and plasticity using hippocampal slices from senescence-accelerated mouse prone 8 (SAMP8) which shows age-related impairment of learning and memory. Oral administration of ManNAc for 8 weeks improved the field excitatory postsynaptic potentials (fEPSPs) in both SAMP8 and SAM resistant 1 (SAMR1), the control strain of SAMP8, at 14 months of age, but not at 6 months of age. On the other hand, ManNAc administration improved long-term potentiation (LTP), representative of long-term synaptic plasticity, of 6 month-old SAMP8 but not of age-matched SAMR1. In addition, ManNAc improved LTP of 14 month-old SAMR1 but not of age-matched SAMP8. At the same time, we checked the PPR but ManNAc did not affect the PPRs at either before or after high-frequency stimulation for LTP induction. These results indicate that chronic administration of ManNAc improves the age-dependent attenuation of synaptic transmission and LTP, and shows the availability of ManNAc treatment as potential therapeutic application for cognitive dysfunction. [5] |
Solubility Data
| Solubility (In Vitro) | H2O : ~125 mg/mL (~565.07 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 25 mg/mL (113.01 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5206 mL | 22.6030 mL | 45.2059 mL | |
| 5 mM | 0.9041 mL | 4.5206 mL | 9.0412 mL | |
| 10 mM | 0.4521 mL | 2.2603 mL | 4.5206 mL |