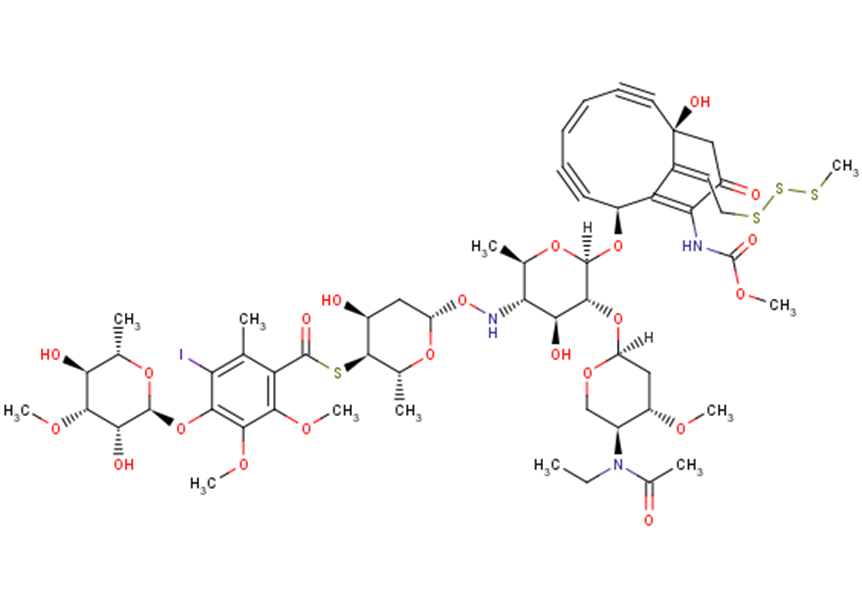
N-Acetyl-Calicheamicin (N-Acetyl-Calicheamicin γ; N-Acetyl-γ-calicheamicin), the N-acetylated analog of calicheamicin, is a novel and potent antitumor antibiotic of the enediyne class. It could be used as a potential warhead (cytotoxic DNA-binding agent) in antibody-drug-conjugation (ADC). Calicheamicins are naturally occurring hydrophobic enediyne antibiotics found in the actinomycete Micromonospora echinospora calichensis. Calicheamicins act by binding to DNA in the minor groove, and causing strand scission.
Physicochemical Properties
| Molecular Formula | C57H76IN3O22S4 |
| Molecular Weight | 1410.38472557068 |
| Exact Mass | 1409.28 |
| Elemental Analysis | C, 48.54; H, 5.43; I, 9.00; N, 2.98; O, 24.96; S, 9.09 |
| CAS # | 108212-76-6 |
| Related CAS # | Calicheamicin;108212-75-5 |
| PubChem CID | 134819845 |
| Appearance | White to off-white solid powder |
| LogP | 2 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 27 |
| Rotatable Bond Count | 24 |
| Heavy Atom Count | 87 |
| Complexity | 2640 |
| Defined Atom Stereocenter Count | 19 |
| SMILES | CCN([C@H]1CO[C@H](C[C@@H]1OC)O[C@@H]2[C@H]([C@@H]([C@H](O[C@H]2O[C@H]3C#C/C=C\C#C[C@@]4(CC(=O)C(=C3C4=CCSSSC)NC(=O)OC)O)C)NO[C@H]5C[C@@H]([C@@H]([C@H](O5)C)SC(=O)C6=C(C(=C(C(=C6OC)OC)O[C@H]7[C@@H]([C@@H]([C@H]([C@@H](O7)C)O)OC)O)I)C)O)O)C(=O)C |
| InChi Key | WPDOZYZAJKUVRZ-DPACUSKXSA-N |
| InChi Code | InChI=1S/C57H76IN3O22S4/c1-13-61(30(6)62)32-25-76-37(23-36(32)71-7)81-50-45(66)42(27(3)78-55(50)80-35-18-16-14-15-17-20-57(70)24-34(64)43(59-56(69)75-11)40(35)31(57)19-21-85-87-84-12)60-83-38-22-33(63)52(29(5)77-38)86-53(68)39-26(2)41(58)48(51(74-10)47(39)72-8)82-54-46(67)49(73-9)44(65)28(4)79-54/h14-15,19,27-29,32-33,35-38,42,44-46,49-50,52,54-55,60,63,65-67,70H,13,21-25H2,1-12H3,(H,59,69)/b15-14-,31-19-/t27-,28+,29-,32+,33+,35+,36+,37+,38+,42-,44+,45+,46-,49-,50-,52-,54+,55+,57+/m1/s1 |
| Chemical Name | S-((2R,3S,4S,6S)-6-((((2R,3S,4S,5R,6R)-5-(((2S,4S,5S)-5-(N-ethylacetamido)-4-methoxytetrahydro-2H-pyran-2-yl)oxy)-4-hydroxy-6-(((2S,5Z,9R,13Z)-9-hydroxy-12-((methoxycarbonyl)amino)-13-(2-(methyltrisulfanyl)ethylidene)-11-oxobicyclo[7.3.1]trideca-1(12),5-dien-3,7-diyn-2-yl)oxy)-2-methyltetrahydro-2H-pyran-3-yl)amino)oxy)-4-hydroxy-2-methyltetrahydro-2H-pyran-3-yl) 4-(((2S,3R,4R,5S,6S)-3,5-dihydroxy-4-methoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-3-iodo-5,6-dimethoxy-2-methylbenzothioate |
| Synonyms | N-Acetyl calicheamicin; N-Acetyl-Calicheamicin; 108212-76-6; N-Acetyl-γ-calicheamicin; N-Acetylcalicheamicin γ; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Anti-tumor antibiotic; ADC cytotoxin |
| References | Cancer Treat Rev. 2008 Feb;34(1):49-60. |
| Additional Infomation | Gemtuzumab ozogamicin (GO) is a chemotherapeutic agent that consists of a humanized anti-CD33 antibody (hP67.6) linked to N-acetyl-calicheamicin 1,2-dimethyl hydrazine dichloride, a potent enediyne antitumor antibiotic. GO was approved conditionally by the Federal Drug Administration in May 2000 as single-agent therapy for first recurrence of acute myeloid leukemia (AML) in patients over the age of 60 years who are unfit for conventional cytotoxic therapy. In this setting, it produces a complete response (CR) rate of 13%, with another 13% achieving CR with inadequate platelet recovery (CRp). The most common adverse effects associated with GO are infusion-related reactions and myelosuppression. GO monotherapy at the dose of 9 mg/m(2) is complicated with hepatic veno-occlusive disease in approximately 5% of cases, particularly prior to or following stem cell transplantation. Attenuated doses of GO or fractionated doses appear to be equally effective and better tolerated. GO has shown remarkable activity in acute promyelocytic leukemia, particularly for the elimination of minimal residual disease. Combinations of GO with chemotherapy as induction or post-remission therapy are promising, and phase III trials are ongoing.[1] |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~70.90 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7090 mL | 3.5451 mL | 7.0902 mL | |
| 5 mM | 0.1418 mL | 0.7090 mL | 1.4180 mL | |
| 10 mM | 0.0709 mL | 0.3545 mL | 0.7090 mL |