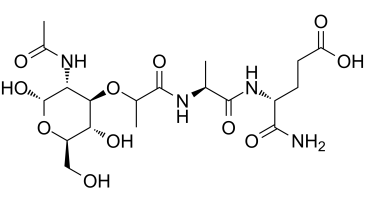
Muramyl Dipeptide (MDP) is a novel and potent NOD2 agonist. It is a synthetic peptidoglycan immunoadjuvant that also causes arthritis and stimulates cellular and humorally bioavailable immunity. It has been demonstrated that NOD2 can recognize MDP, but not TLR2, TLR2/1, or TLR2/6 associations [1, 2]. There is no reaction to the D-D or L-L analogs in this recognition, which is highly stereospecific for the L-D isomer.
Physicochemical Properties
| Molecular Formula | C19H34N4O10 |
| Molecular Weight | 492.47800 |
| Exact Mass | 492.206 |
| Elemental Analysis | C, 47.69; H, 7.16; N, 11.71; O, 33.44 |
| CAS # | 53678-77-6 |
| Related CAS # | 53678-77-6 |
| PubChem CID | 451714 |
| Sequence | N-Acetylmuramyl-L-alanyl-D-isoglutamine |
| Appearance | White to off-white solid powder |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 1023.8±65.0 °C at 760 mmHg |
| Flash Point | 572.9±34.3 °C |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.578 |
| LogP | -2.97 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 34 |
| Complexity | 765 |
| Defined Atom Stereocenter Count | 8 |
| SMILES | C[C@H](NC([C@H](O[C@@H]1[C@@H](NC(C)=O)C(O[C@@H]([C@H]1O)CO)O)C)=O)C(N[C@@H](C(N)=O)CCC(O)=O)=O |
| InChi Key | BSOQXXWZTUDTEL-QAQREVAFSA-N |
| InChi Code | InChI=1S/C19H32N4O11/c1-7(17(30)23-10(16(20)29)4-5-12(26)27)21-18(31)8(2)33-15-13(22-9(3)25)19(32)34-11(6-24)14(15)28/h7-8,10-11,13-15,19,24,28,32H,4-6H2,1-3H3,(H2,20,29)(H,21,31)(H,22,25)(H,23,30)(H,26,27)/t7-,8+,10+,11+,13+,14+,15+,19?/m0/s1 |
| Chemical Name | (4R)-4-[[(2S)-2-[[(2R)-2-[(3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxypropanoyl]amino]propanoyl]amino]-5-amino-5-oxopentanoic acid |
| Synonyms | Adjuvant Peptide; Muramyl Dipeptide; N-Acetylmuramyl-L-alanyl-D-isoglutamine; Adjuvant Peptide; 53678-77-6; N-Acetylmuramyl-L-alanyl-D-isoglutamine; CHEMBL1779325; (4R)-4-[[(2S)-2-[[(2R)-2-[(3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxypropanoyl]amino]propanoyl]amino]-5-amino-5-oxopentanoic acid; (4R)-4-[[(2S)-2-[[(2R)-2-[(2S,3R,4R,5S,6R)-3-Acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxypropanoyl]amino]propanoyl]amino]-5-amino-5-oxopentanoic acid; MFCD00077638; Ac-muramyl-Ala-D-Glu-NH2 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | p38 MAPK; NLRP1 |
| ln Vitro | MDP directly augments osteoblast differentiation and bone-forming gene expression by Runx2 activation. MDP has no direct effect, but it indirectly inhibits osteoclast differentiation by lowering the RANKL/OPG ratio. The MDP receptor, Nod2, is expressed more frequently when MDP is present, and Nod2-deficient individuals do not experience MDP-induced bone formation or osteoblast activation. [1] |
| ln Vivo | Muramyl dipeptide (MDP)-treated mice show increased bone and mineral density due to enhanced bone formation. Surprisingly, pre- or post-treatment with MDP reduces bone loss in mouse models of RANKL-induced osteoporosis.[1] |
| Enzyme Assay |
Enzyme-linked immunosorbent assay[1] The levels of OPG and RANKL in culture supernatant and bone marrow extracellular fluid were measured using Quantikine enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions. Real-time reverse transcriptase–polymerase chain reaction[1] The mRNA expression of ALP, BSP, Runx2, Nod2, and GAPDH in osteoblasts was determined by using real-time reverse transcriptase–polymerase chain reaction (real-time RT-PCR) as described.28 The sequences of each primer are as follows: ALP: forward 5′-CCAACTCTTTTGTGCCAGAGA-3′ and reverse 5′-GGCTACATTGGTGTTGAGCTTTT-3′; BSP, forward 5′-GAATGCTGTGTCCTCTGAAG-3′ and reverse 5′-AATCCTCGCTCTCTGCATGG-3′; Runx2: forward 5′-AACGATCTGAGATTTGTGGGC-3′ and reverse 5′-CCTGCGTGGGATTTCTTGGTT-3′; Nod2: forward 5′-CCTGGTACGTGCCCAAAGTAG-3′ and reverse 5′-GCCAAGTAGAAAGCGGCAAA-3′; and GAPDH: forward 5′-AGGTCGGTGTGAACCGGATTTG-3′ and reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′. |
| Cell Assay | In 48-well plates with 2×104 cells/400 l per well, MC3T3-E1 cells, BMSCs, or primary osteoblast precursors from mouse calvaria are plated before being incubated with osteoblast induction medium in the absence or presence of MDP. Every two days, half of the medium is replaced with brand-new osteoblast induction medium. At days 6 and 12, the cells are stained with alizarin red S to detect bone mineralization and with ALP to detect osteoblast differentiation. |
| Animal Protocol |
Five-week-old C57BL/6 mice, one-day-old neonatal mice, B6.129S1-Nod2tm1Flv/J mice 1.25 mg/kg IP Micro–computed tomography[1] Mice acclimated for 1 week were intraperitoneally administered with 200 µL of 1.25 mg/kg MDP (n = 5) or 200 µL PBS (n = 5) at days 0 and 4. At day 7 after initial administration, the femurs were removed from mice and fixed in 10% formalin. The bones were scanned using X-ray micro–computed tomography (μCT) at 70 kV, 142 mA, 10 W, 0.5 mm Aluminium filter, and 7 µm per pixel scan resolution. The μCT images were reconstructed by the SkyScan NRecon program and analyzed using the SkyScan Dataviewer 1.3.2 and SkyScan CT analyzer software version 1.8.1.5. Three-dimensional (3D) images were created by SkyScan CT volume version 2.0. To quantitatively analyze the trabecular bone, 144 slides starting from 144 sections above the distal growth plate were selected and the region of interest of trabecular bone was defined. The trabecular bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) were calculated from voxel-based 3D reconstruction of μCT images. Calcein double labeling and calculation of mineral apposition rate[1] Mice were intraperitoneally administered with 20 mg/kg of calcein 1 day before the first administration of MDP at 1.25 mg/kg or PBS at days 1 and 5. The mice were intraperitoneally administered with 20 mg/kg of calcein again 1 day after the last MDP administration. At day 9 after the first injection with MDP, the femurs were fixed and embedded in methyl methacrylate. The resin blocks were sectioned and the calcein-labeled sections were observed using confocal microscopy . The mineral apposition rate (MAR) is the distance between the midpoints of two labels. Mineralizing surface/bone surface (MS/BS), MAR, and bone formation rate (BFR) were quantified using the OsteoMeasure software |
| References |
[1]. Muramyl Dipeptide, a Shared Structural Motif of Peptidoglycans, Is a Novel Inducer of Bone Formation through Induction of Runx2. J Bone Miner Res. 2017 Jul;32(7):1455-1468. [2]. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015 Oct;22(10):1676-86. |
| Additional Infomation |
Muramyl dipeptide is a glycopeptide that is N-propionyl-L-alanyl-D-alpha-glutamine in which the pro-R hydrogen of the propionyl group has been replaced by the oxygen at position 3 of 2-acetamido-2-deoxy-beta-D-glucopyranose. A peptidoglycan constituent of both Gram-positive and Gram-negative bacteria. It has a role as an immunological adjuvant. It is a conjugate acid of a N-acetyl-beta-D-muramoyl-L-alanyl-D-isoglutamine(1-). Muramyl Dipeptide is a naturally occurring component of bacterial cell walls that has the capacity to activate macrophages. Peptidoglycan immunoadjuvant originally isolated from bacterial cell wall fragments; also acts as pyrogen and may cause arthritis; stimulates both humoral and cellular immunity. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 98~260 mg/mL (199~527.9 mM) Ethanol: ~98 mg/mL (~199 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 6.5 mg/mL (13.20 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 65.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 6.5 mg/mL (13.20 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 65.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 6.5 mg/mL (13.20 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 65.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0305 mL | 10.1527 mL | 20.3054 mL | |
| 5 mM | 0.4061 mL | 2.0305 mL | 4.0611 mL | |
| 10 mM | 0.2031 mL | 1.0153 mL | 2.0305 mL |