Mupirocin (BRL-4910A; Pseudomonic acid) is an potent isoleucyl t-RNA synthetase inhibitor and an orally bioactive antibiotic isolated from Pseudomonas fluorescens. It is used in the treatment of bacterial skin infections. Mupirocin is an antibiotic of the monoxycarbolic acid class. The effect of Mupirocin is concentration dependent, being bacteriostatic at low concentrations and bactericidal at high concentrations. It is used topically and is effective against Gram-positive bacteria, including MRSA. Mupirocin is a mixture of several pseudomonic acids, with pseudomonic acid A (PA-A) constituting greater than 90% of the mixture.
Physicochemical Properties
| Molecular Formula | C52H90CAO20 |
| Molecular Weight | 1077.35288 |
| Exact Mass | 1074.57 |
| Elemental Analysis | C, 58.08; H, 8.44; Ca, 3.73; O, 29.76 |
| CAS # | 115074-43-6 |
| Related CAS # | Mupirocin;12650-69-0;Mupirocin calcium;104486-81-9 |
| PubChem CID | 446596 |
| Appearance | White to off-white solid powder |
| Boiling Point | 672.3ºC at 760 mmHg |
| Melting Point |
77-78 77 - 78 °C |
| Flash Point | 216.5ºC |
| Vapour Pressure | 5.91E-21mmHg at 25°C |
| LogP | 4.902 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 17 |
| Heavy Atom Count | 35 |
| Complexity | 694 |
| Defined Atom Stereocenter Count | 8 |
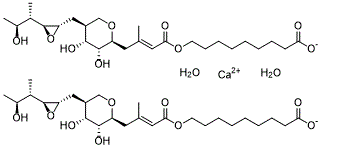
| SMILES | O.O.[Ca+2].OC(CCCCCCCCOC(/C=C(/CC1OCC(CC2OC2C(C(O)C)C)C(O)C1O)\C)=O)=O.OC(CCCCCCCCOC(/C=C(/CC1OCC(CC2OC2C(C(O)C)C)C(O)C1O)\C)=O)=O |
| InChi Key | DDHVILIIHBIMQU-YJGQQKNPSA-L |
| InChi Code | InChI=1S/2C26H44O9.Ca.2H2O/c2*1-16(13-23(30)33-11-9-7-5-4-6-8-10-22(28)29)12-20-25(32)24(31)19(15-34-20)14-21-26(35-21)17(2)18(3)27/h2*13,17-21,24-27,31-32H,4-12,14-15H2,1-3H3,(H,28,29)2*1H2/q+2/p-2/b2*16-13+/t2*17-,18-,19-,20-,21-,24+,25-,26-/m00.../s1 |
| Chemical Name | calcium 9-(((E)-4-((2S,3R,4R,5S)-3,4-dihydroxy-5-(((2S,3S)-3-((2S,3S)-3-hydroxybutan-2-yl)oxiran-2-yl)methyl)tetrahydro-2H-pyran-2-yl)-3-methylbut-2-enoyl)oxy)nonanoate dihydrate |
| Synonyms | Mupirocin calcium salt; Calcium Mupirocin Dihydrate; Mupirocin Calcium Hydrate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro |
With MIC values ranging from 0.06-0.25 μg/mL (MIC50 = 0.12 μg/mL, MIC90 = 0.25 μg/mL), mupirocin (BRL-4910A, pseudomonoic acid) calcium hydrate (0-100 μM; 48 h) exhibits antibacterial activity against staphylococci, streptococci, and some gram-negative bacteria[1].
The presence of human serum inhibits the activity of mugirocin calcium hydrate due to its strong 95% binding to human serum protein[1]. It appears that the antimicrobial activity of mugirocin calcium hydrate is achieved by reversibly inhibiting isoleucyl-transfer RNA, which in turn inhibits the synthesis of bacterial proteins and RNA[2]. Mupirocin calcium hydrate (2% ointment) decreases the expression of tumor necrosis factor-alpha (TNF-α), increases the leavel of vascular endothelial growth factor (VEGF), and decreases the levels of pro-inflammatory cytokines IL-1β and IL-17[4]. With MICs of 0.25, 1.26, and 1.59 mg/L, mugirocin calcium hydrate inhibits MS (S. epidermidis ATCC 12228), MR (S. epidermidis (Se56-99)), and VIR (S. epidermidis (Se43-98))[5]. |
| ln Vivo |
MRSA: Staphylococcus aureus resistant to meticillin Following oral and parenteral administration, mupirocin (BRL-4910A, pseudomononic acid) calcium is well absorbed; however, the prolonged breakdown of the antibiotic to the antibacterially inactive metabolite monic acid A resulted in short-lived serum antibiotic concentrations [1]. Using either topical treatment, mugirocin calcium (2% ointment; external administration; twice daily; 3-6 d) reduces the overall bacterial loads in the skin lesions[3]. Mice with pressure ulcers infected with MRSA are treated with 2% ointment of mupirocin calcium administered externally every 4 days[4]. Staphylococcus epidermidis-caused vascular prosthetic graft infection can be prevented with mugirocin calcium (100 mg/mL; s.c.; 7 d)[5]. |
| Cell Assay |
Cell Line: Staphylococcus aureus Concentration: 0-100 μM/mL Incubation Time: 24, 48 hours Result: produced a 90–99% reduction at 24 hours, with MIC and MBC values ranging from 0.12-1.0 μM/mL and 4.0–32 μM/mL, respectively, at 48 hours. |
| Animal Protocol |
Animal Model: MRSA skin infection model in mice (10-12 weeks old)[3] Dosage: 2% ointment Administration: External administration; twice daily; 3-6 days Result: decreased the overall amount of bacteria present in the skin lesions, with reductions of 2.0 and 5.1 log10 CFU on days three and six, respectively. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Systemic or percutaneous absorption of mupirocin following dermal application is expected to be minimal in adults and children. Occlusive dressings do not significantly enhance drug absorption, but damaged skin may allow enhanced penetration of the drug across the skin barrier. Any mupirocin reaching the systemic circulation is rapidly metabolized to form the inactive monic acid, which is eliminated by renal excretion. Following the application of Centany (mupirocin ointment),2% to a 400 cm2 area on the back of 23 healthy volunteers once daily for 7 days, the mean (range) cumulative urinary excretion of monic acid over 24 hrs following the last administration was 1.25% (0.2% to 3.0%) of the administered dose of mupirocin. No information available. No information available. Metabolism / Metabolites Following intravenous or oral administration, mupirocin undergoes rapid hepatic metabolism to form the principal metabolite monic acid, which has no antibacterial activity. Biological Half-Life In healthy male volunteers, the elimination half-life of mupirocin was about 20 to 40 minutes following intravenous administration. The elimination half-life of monic acid was about 30 to 80 minutes. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because less than 1% is absorbed after topical application, mupirocin is considered a low risk to the nursing infant.[1] Ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.[2] Mupirocin applied topically to the nipples appears to be relatively ineffective as a treatment for sore, cracked nipples. ◉ Effects in Breastfed Infants A mother who was exclusively nursing her 52-day-old infant developed a soft-tissue infection. She was treated with intravenous teicoplanin 400 mg every 12 hours for 3 doses, then 400 mg daily for 5 days total, intravenous ceftriaxone 1 gram daily, topical mupirocin cream twice daily. A careful follow-up indicated that her infant had no adverse effects.[3] ◉ Effects on Lactation and Breastmilk A small, randomized, unblinded trial of mothers with sore, cracked nipples was performed. Mupirocin 2% applied to the nipples after each feeding was much less effective (16% vs 79%) than an oral antibiotic (cloxacillin or erythromycin for 10 days) in resolving the problem. Additionally, more patients' condition worsened (28% vs 5%) with mupirocin than with an oral antibiotic.[4] In a randomized, double-bind trial, lanolin was compared to an all-purpose nipple ointment containing mupirocin 1%, betamethasone 0.05%, and miconazole 2% for painful nipples while nursing in the first 2 weeks postpartum. The two treatments were equally effective in reducing nipple pain, nipple healing time, breastfeeding duration, breastfeeding exclusivity rate, mastitis and nipple symptoms, side effects or maternal satisfaction with treatment.[5] Protein Binding The protein binding of mupirocin is reported to be over 95%. |
| References |
[1]. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. 1985 Apr;27(4):495-8. [2]. Mupirocin: a topical antibiotic with a unique structure and mechanism of action. Clin Pharm. 1987 Oct;6(10):761-70. [3]. Efficacy of topical and systemic antibiotic treatment of meticillin-resistant Staphylococcus aureus in a murine superficial skin wound infection model. Int J Antimicrob Agents. 2013 Sep. 42(3):272-5. [4]. Mohammad H, Abutaleb NS, Dieterly AM, Lyle LT, Seleem MN. Investigating auranofin for the treatment of infected diabetic pressure ulcers in mice and dermal toxicity in pigs. Sci Rep. 2021 May 25;11(1):10935. [5]. Mupirocin prophylaxis against methicillin-susceptible, methicillin-resistant, or vancomycin-intermediate Staphylococcus epidermidis vascular-graft infection. Antimicrob Agents Chemother. 2000 Oct. 44(10):2842-4. |
| Additional Infomation |
Mupirocin is an alpha,beta-unsaturated ester resulting from the formal condensation of the alcoholic hydroxy group of 9-hydroxynonanoic acid with the carboxy group of (2E)-4-[(2S)-tetrahydro-2H-pyran-2-yl]-3-methylbut-2-enoic acid in which the tetrahydropyranyl ring is substituted at positions 3 and 4 by hydroxy groups and at position 5 by a {(2S,3S)-3-[(2S,3S)-3-hydroxybutan-2-yl]oxiran-2-yl}methyl group. Originally isolated from the Gram-negative bacterium Pseudomonas fluorescens, it is used as a topical antibiotic for the treatment of Gram-positive bacterial infections. It has a role as a bacterial metabolite, an antibacterial drug and a protein synthesis inhibitor. It is a monocarboxylic acid, a member of oxanes, an epoxide, a secondary alcohol, a triol and an alpha,beta-unsaturated carboxylic ester. It is a conjugate acid of a mupirocin(1-). Mupirocin, formerly termed pseudomonic acid A, is a novel antibacterial agent with a unique chemical structure and mode of action apart from other antibiotic agents. Produced by fermentation using the organism Pseudomonas fluorescens, mupirocin is a naturally-occurring antibiotic that displays a broad-specturm activity against many gram-positive bacteria and certain gram-negative bacteria in vitro. It primarily works by inhibiting bacterial protein synthesis. Due to its unique mode of action of inhibiting the activity of bacterial isoleucyl-tRNA synthetase, mupirocin does not demonstrate cross-resistance with other classes of antimicrobial agents, giving it a therapeutic advantage. It is available in topical formulations only due to extensive systemic metabolism and is used in the treatment of impetigo caused by Staphylococcus aureus and Streptococcus pyogenes and traumatic skin lesions due to secondary skin infections caused by S. aureus and S. pyogenes. There is also some clinical evidence that suggests the potential role of mupirocin in eradicating nasal carriage of Staphylococci when administered intranasally. Mupirocin is commonly marketed under the brand name Bactroban. Mupirocin is a RNA Synthetase Inhibitor Antibacterial. The mechanism of action of mupirocin is as a RNA Synthetase Inhibitor. Mupirocin has been reported in Pseudomonas fluorescens with data available. Mupirocin is a natural crotonic acid derivative extracted from Pseudomonas fluorescens. Mupirocin inhibits bacterial protein synthesis by specific reversible binding to bacterial isoleucyl tRNA synthase. With excellent activity against gram-positive staphylococci and streptococci, it is primarily used for treatment of primary and secondary skin disorders, nasal infections, and wound healing. (NCI04) A topically used antibiotic from a strain of Pseudomonas fluorescens. It has shown excellent activity against gram-positive staphylococci and streptococci. The antibiotic is used primarily for the treatment of primary and secondary skin disorders, nasal infections, and wound healing. Drug Indication Indicated for the treatment of impetigo and secondary skin infections, leading to traumatic skin lesions, due to _Staphylococcus aureus_ and _Streptococcus pyogenes_. Mechanism of Action Mupirocin specifically and reversibly binds to bacterial isoleucyl transfer-RNA (tRNA) synthetase, which is an enzyme that promotes the conversion of isoleucine and tRNA to isoleucyl-tRNA. Inhibition of this enzyme subsequently leads to the inhibition of the bacterial protein and RNA synthesis. Mupirocin is bacteriostatic at lower concentrations but it exerts bactericidal effects with prolonged exposure, killing 90-99% of susceptible bacteria over a 24 hour period. Pharmacodynamics Mupirocin is reported to be active against susceptible aerobic gram-positive cocci, such as _Staphylococcus aureus_, _Staphylococcus epidermidis_, and other beta-hemolytic streptococci_Streptococcus pyogenes_. It mediates its antibacterial activity by inhibiting the bacterial protein synthesis and formation of bacterial proteins essential for survival. The minimum bactericidal concentration (MBC) against relevant pathogens is generally eight-fold to thirty-fold higher than the minimum inhibitory concentration (MIC). In one clinical study investigating the therapeutic effectiveness of topical mupirocin in impetigo, the therapeutic response rate was about 94 to 98% after one week following the end of therapy. In clinical studies of patients with primary and secondary skin infections, both elimination of the bacterial pathogen and clinical cure or improvement hav been demonstrated in over 90% of patients receiving topical mupirocin. Mupirocin resistance as high as 81% has been reported previously. Resistance to mupirocin, which occurs more frequently in methicillin-resistant than methicillin-susceptible staphylococci, may occur with the production of a modified isoleucyl-tRNA synthetase, or the acquisition of, by genetic transfer, a plasmid mediating a new isoleucyl-tRNA synthetase. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~185.64 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.25 mg/mL (2.32 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 12.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.25 mg/mL (2.32 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 12.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1.25 mg/mL (2.32 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 12.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9282 mL | 4.6410 mL | 9.2820 mL | |
| 5 mM | 0.1856 mL | 0.9282 mL | 1.8564 mL | |
| 10 mM | 0.0928 mL | 0.4641 mL | 0.9282 mL |