Physicochemical Properties
| Molecular Formula | C22H22O4 |
| Molecular Weight | 350.41 |
| Exact Mass | 350.151 |
| CAS # | 1393922-01-4 |
| PubChem CID | 66573474 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.139±0.06 g/cm3(Predicted) |
| Boiling Point | 528.2±50.0 °C(Predicted) |
| LogP | 4.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 26 |
| Complexity | 542 |
| Defined Atom Stereocenter Count | 0 |
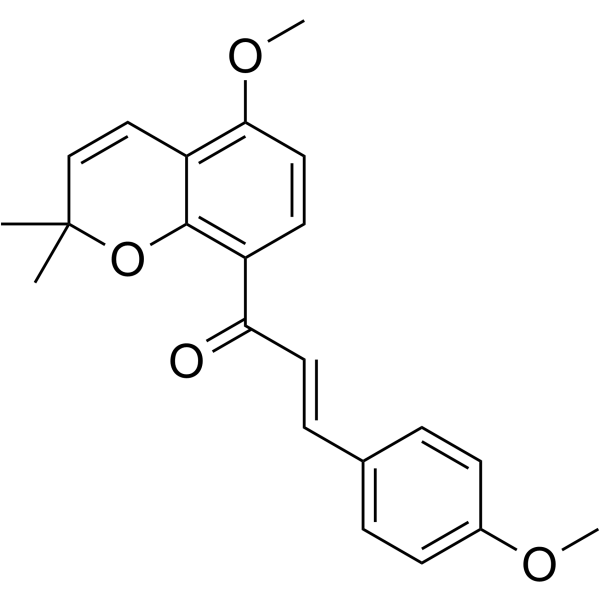
| SMILES | CC1(C=CC2=C(C=CC(=C2O1)C(=O)/C=C/C3=CC=C(C=C3)OC)OC)C |
| InChi Key | GZUOKKIDYHPTAZ-YRNVUSSQSA-N |
| InChi Code | InChI=1S/C22H22O4/c1-22(2)14-13-18-20(25-4)12-10-17(21(18)26-22)19(23)11-7-15-5-8-16(24-3)9-6-15/h5-14H,1-4H3/b11-7+ |
| Chemical Name | (E)-1-(5-methoxy-2,2-dimethylchromen-8-yl)-3-(4-methoxyphenyl)prop-2-en-1-one |
| Synonyms | Millepachine; 1393922-01-4; (E)-1-(5-methoxy-2,2-dimethylchromen-8-yl)-3-(4-methoxyphenyl)prop-2-en-1-one; (E)-1-(5-Methoxy-2,2-dimethyl-2H-chromen-8-yl)-3-(4-methoxyphenyl)prop-2-en-1-one; 1-(5-Methoxy-2,2-dimethyl-2H-chromen-8-yl)-3-(4-methoxyphenyl)prop-2-en-1-one; 1579976-20-7; CHEMBL2041121; SCHEMBL16697961; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Natural chalcone; tubulin/microtubule; anticancer |
| ln Vitro | Millepachine (1.25–20 μM; 48 h) significantly reduces the growth of A2780CP cells that are resistant to cisplatin[1]. Apoptosis and G2/M arrest are induced in cisplatin-sensitive A2780S and cisplatin-resistant A2780CP cells by millepachine (2–8 μM) within 24 or 48 hours[1]. In A2780S and A2780CP cells, millepachine (2–8 μM; 24 h) reduces topoisomerase II levels[1]. In A2780CP cells, millepachine (2–8 μM; 24 h) suppresses the activity of the ATP-binding cassette transporter [1]. |
| ln Vivo |
Mice exposed to Millepachine (MIL) (20 mg/kg; intravenously every two days for 14 days) do not develop tumors[1]. In an excised A2780S xenograft model, millepachine (20 mg/kg; iv every two days for 14 days) did not result in acquired drug resistance[1]. Millepachine (MIL) inhibited tumor growth in the cisplatin-resistant A2780CP xenograft model [1] Platinum-based chemotherapy is the first-line chemotherapy for the clinical treatment of human ovarian cancer. In vivo assay results revealed that 5 mg/kg DDP could significantly inhibit tumor growth in the A2780S xenograft model after two times injection (p < 0.001; Figure 5a), and compared with vehicle control treatment, it significantly reduced tumor volume (995.83 ± 160.75 vs. 2805.02 ± 354.95 mm3; p < 0.001; Figure 5a) and weight (0.965 ± 0.276 vs. 3.10 ± 0.152 g; p < 0.001; Figure 5a) with the inhibitory rate of 68.92%. And compared with the vehicle control, Millepachine (MIL) (20 mg/kg) also significantly reduced tumor volume (896.26 ± 226.27 mm3; p < 0.001) and tumor weight (0.832 ± 0.097 g; p < 0.001) after seven times injection (Figure 5a) with the inhibitory rate of 73.21%, demonstrating comparable antitumor activity to 5 mg/kg DDP. In the cisplatin-resistant A2780CP xenograft model, 5 mg/kg DDP exhibited minimal inhibition of tumor growth after two times treatment, but 20 mg/kg Millepachine (MIL) could still significantly inhibit tumor growth after six times treatment. Compared with the tumor volume in the vehicle control (3481.94 ± 277.71 mm3) and 5 mg/kg DDP-treated group (2452.90 ± 386.52 mm3), the tumor volume in the group treated with 20 mg/kg Millepachine (MIL) was remarkably reduced (1069.09 ± 213.70 mm3; MIL vs. control, p < 0.001; MIL vs. DDP, p < 0.01; Figure 5b). The tumor weight was 3.448 ± 0.230 g in vehicle control group, 2.246 ± 0.101 g in 5 mg/kg DDP-treated group, and 1.213 ± 0.094 g in 20 mg/kg MIL-treated group, respectively (MIL vs. control, p < 0.001; MIL vs. DDP, p < 0.001; Figure 5b). The inhibitory rate of 20 mg/kg MIL treatment was 65.58%, which was similar to that in A2780S xenograft model, indicating that MIL exhibited good antitumor activity both in cisplatin-sensitive and cisplatin-resistant human ovarian cancer in vivo. Drug resistance may be either preexistent (intrinsic resistance) or induced by drugs (acquired drug resistance; Lippert, Ruoff, & Volm, 2008). Many anticancer drug candidates encounter problems associated with development-acquired drug resistance (Hambley & Hait, 2009; Heddleston et al., 2010). In this study, we investigated whether Millepachine (MIL) would develop acquired drug resistance in an excised A2780S xenograft model. Upon a second round of seven times treatment, MIL significantly reduced the tumor volume (895.93 ± 1660.15 vs. 2129.51 ± 312.70 mm3; p < 0.001) and tumor weight (0.85 ± 0.99 vs. 2.12 ± 0.15 g; p < 0.001; Figure 5c) with tumor inhibitory rate of 60.03% compared with the vehicle control, suggesting that MIL did not induce acquired drug resistance in A2780S tumors after noncontinuous 28 days of treatment (about 1 week off for the growth of tumors). In addition, compared with the vehicle control group, the BALB/c nu/nu mice treated with 5 mg/kg DDP rather than those treated with 20 mg/kg Millepachine (MIL) exhibited an obvious reduction in the body weight (DDP vs. control, p < 0.001; DDP vs. MIL, p < 0.001; Figure 5d). These results demonstrated that 20 mg/kg Millepachine (MIL) showed higher safety than 5 mg/kg DDP in vivo. These results suggested that Millepachine (MIL) is a highly efficient antitumor candidate with low toxicity in vivo. |
| Cell Assay |
Cell Proliferation Assay[1] Cell Types: A2780CP cells Tested Concentrations: 0, 1.25, 2.5, 5, 10, 20 μM Incubation Duration: 48 hrs (hours) Experimental Results: Inhibited the cells proliferation with an IC50 of 4 μM. Cell Cycle Analysis[1] Cell Types: A2780S and A2780CP cells Tested Concentrations: 2, 4, 8 μM Incubation Duration: 24 or 48 hrs (hours) Experimental Results: Induced significant G2/M arrest both in both cells. The percentage of cells in the G2/M fraction increased from 15.99% in vehicle cells to 24.93%, 60.67%, and 77.31% at dose of 2, 4, and 8 μM in A2780S cells, . Apoptosis Analysis[1] Cell Types: A2780S and A2780CP cells Tested Concentrations: 2, 4, 8 μM Incubation Duration: 24 or 48 hrs (hours) Experimental Results: The percentage of apoptotic cells increased from 1.49% in vehicle cells to 10.98%, 20.60%, respectively. and 39.43% at dose of 2, 4, and 8 μM in A2780S cells, respectively. The percentage of apoptotic cells increased from 0.87% to 10.97%, 25.28%, and 37.59% in A2780CP cells, respectively. Western Blot Analysis[1] Cell Types: A2780S and A2780CP cells Concen Cell proliferation assay [1] Cells were seeded in 96-well plates and cultured overnight. Then, cells were treated with different concentrations of Millepachine (MIL) or DDP (0, 1.25, 2.5, 5, 10, and 20 μM) for 48 hr. Cell viability was determined using the XTT assay. The absorbance at 450 nm was determined in each well using the Spectramax M5 Microtiter Plate Luminometer. Flow cytometry [1] After different treatment modalities, cells were stained with PI to analyze cell cycle distribution or Annexin V-FITC and PI to measure apoptotic cells (Annexin V+/PI−) using a flow cytometer. Western blot [1] After treatment with various concentrations (0, 2, 4, and 8 μM) of Millepachine (MIL) or DDP (2.5 or 10 μM) for 24 hr, cells were lysed in cell lysis buffer on ice. The protein concentration was determined by Bio-Rad protein assay. Briefly, 30 μg of each sample was fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane. The protein bands were probed with antibodies against topoisomerase IIα (TOPO II), Pgp, MRP1, and MRP2 (Abcam, UK) overnight at 4°C. Blots were incubated in secondary antibodies for 1 hr at room temperature. Proteins were visualized with enhanced chemiluminescence. Immunofluorescence imaging [1] Cells were cultured on poly-l-lysine-coated glass coverslips. After exposure to different treatments, cells were fixed with 4% formaldehyde polymer for 10 min and subsequently incubated with primary Pgp or MRP2 antibody overnight at 4°C. The secondary antibody was incubated for 1 hr at room temperature. The nucleus was stained with Hoechst 33258. The images were detected using an inverted fluorescence microscopy. Rhodamine 123 staining [1] A2780CP cells were treated with various concentrations (0, 2, 4, and 8 μM) of Millepachine (MIL) or 10 μM DDP for 24 hr. Then, cells were stained with Rh123 (10 μl/1 × 106 cells) for 20 min at 37°C in the dark. Cell fluorescence was analyzed using a flow cytometer with fluorescence excited using blue laser (488 nm). Green fluorescence was measured at 530 ± 20 nm. |
| Animal Protocol |
Animal/Disease Models: Female BALB/c nude mice (6 weeks) are injected A2780S or A2780CP cells[1] Doses: 20 mg/kg Route of Administration: Iv every two days for 2-14 days Experimental Results: diminished tumor volume and tumor weight with the inhibitory rate of 73.21% and 65.58% in A2780S (after seven times injection) and A2780CP (after six times injection) xenograft model, respectively. With low toxicity in vivo. In vivo tumor xenograft [1] To determine the effect of Millepachine (MIL) on overcoming cisplatin resistance in human ovarian cancer in vivo, A2780S or A2780CP cells (5 × 106 in 100 μl saline) were injected subcutaneous into the right flanks of female nude mice (6-week-old BALB/c (nu/nu) mice), separately, to establish two human ovarian tumor xenograft models. After 2 weeks, the tumors were grown to about 1,000 mm3 and aseptically dissected and pieces of tumor tissue (2–3 mm3 in size) were transplanted subcutaneous by trocar into mice. When the tumor size reached 100 mm3 (about a week of cultivation), mice were randomly divided into three groups: (a) Control group (n = 6): mice were injected with 100 μl normal saline every 2 days; (b) MIL-treated group (n = 6): mice were treated with 20 mg/kg MIL intravenous (iv) every 2 days; (c) DDP-treated group (n = 6): mice were treated with 5 mg/kg DDP intraperitoneal (ip) every 5 days. The tumor burden of mice was measured every 2 days with a caliper (calculated volume [mm3] = π/6 × Length × Width × Width). An excised A2780S xenograft model was also performed to investigate the antitumor effect of Millepachine (MIL) on tumors with acquired drug resistance. We excised A2780S tumors from null mice after 14 days of treatment with 20 mg/kg MIL when the tumors grew to 800 mm3, they were cut into pieces (2–3 mm3) and implanted again in the right flank of nu/nu mice. When tumor volume reached 100 mm3 (about a week of cultivation), a second round of treatment was initiated with intravenous injection of 20 mg/kg MIL every 2 days. |
| References |
[1]. Millepachine showed novel antitumor effects in cisplatin-resistant human ovarian cancer through inhibiting drug efflux function of ATP-binding cassette transporters. Phytother Res. 2018 Dec; 32(12):2428-2435. |
| Additional Infomation |
Millepachine (MIL), a bioactive natural chalcone from Chinese herbal medicine Millettia pachycarpa Benth, exhibits strong antitumor effects against many human cancer cells both in vitro and in vivo. In this study, we found that MIL significantly inhibited the proliferation of cisplatin-resistant A2780CP cells via inducing obvious G2/M arrest and apoptosis and down-regulating the activity of topoisomerase II protein. We further found that the mechanism by which MIL showed good antitumor effects in cisplatin-resistant human ovarian cancer was associated with inhibiting the expression of ATP-binding cassette transporters in cisplatin-resistant A2780CP cells. Importantly, MIL did not only significantly inhibit the tumor growth in cisplatin-sensitive A2780S xenograft model, with an inhibitory rate of 73.21%, but also inhibited the tumor growth in the cisplatin-resistant A2780CP xenograft model, with an inhibitory rate of 65.68% (p < 0.001 vs. control; p < 0.001 vs. DDP). In addition, MIL did not induce acquired drug resistance in A2780S tumor-bearing mice with an inhibitory rate of 60.03%. The promising in vitro and in vivo performance indicated that MIL exhibited potential significance for drug research and development.[1]
Cispaltin (DDP) is a classic cancer chemotherapeutic agent widely used in the treatment of several types of solid tumors (Florea & Büsselberg, 2011), through inducing DNA damage by targeting DNA (Basu & Krishnamurthy, 2010; Loehrer & Einhorn, 1984). However, its efficiency in cancer treatment is often limited by toxic side effects and tumor resistance (Loehrer & Einhorn, 1984; Stordal, Pavlakis, & Davey, 2007). MIL, a natural chalcone, could also induce DNA damage through inhibiting TOPO II activity against various tumor cells (Wu et al., 2016). In this study, MIL did not only exhibit better antitumor activity than DDP against human ovarian cancer and cisplatin-resistant human ovarian cancer but also demonstrated better safety in vivo (Figure 5). Furthermore, MIL did not induce acquired drug resistance in A2780S tumors (Figure 5c). These results demonstrated that MIL exhibited significant potential to be developed as a novel anticancer candidate. We also discussed the mechanism by which MIL inhibited the proliferation of cisplatin-resistant human ovarian cancer. The most common characteristic of cisplatin-resistant tumor cells is the reduction of intracellular DDP accumulation (Gately & Howell, 1993; Loh, Mistry, Kelland, Abel, & Harrap, 1992). ABC transporter proteins represent one of the largest and oldest families of membrane proteins in all extant phyla from prokaryotes to humans, which couple the energy derived from ATP hydrolysis essentially to translocate toxic compounds and various other substrates across the membrane (Gottesman et al., 2002; Kathawala et al., 2015; Li et al., 2016). Pgp and MRP family proteins as two important types of ABC transporter are expressed in some tumors, providing resistance to chemotherapy agents (Dean, Fojo, & Bates, 2005; Szakács et al., 2006). Some studies report overexpression of ABC transporter proteins in several cisplatin-resistant cancer cells, demonstrating a close link between in ABC transporters and cisplatin resistance (Sun et al., 2016; Tang et al., 2014; Tonigold et al., 2014; Yamasaki et al., 2011). In this study, we found high levels of Pgp, MRP1, and MRP2 proteins in cisplatin-resistant A2780CP cells (Figure 4a). However, MIL reduced Pgp, MRP1, and MRP2 proteins expression in cisplatin-resistant A2780CP cells (Figure 4). Overexpression of ABC transporter proteins is the major mechanism of MDR, which is a key determinant of cancer chemotherapy failure (Gottesman et al., 2002; Szakács et al., 2006). The inhibition of ABC transporters activity by MIL suggests that MIL has the potential to reverse MDR in tumor cells. [1] |
Solubility Data
| Solubility (In Vitro) | DMSO : 100 mg/mL (285.38 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (7.13 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.13 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8538 mL | 14.2690 mL | 28.5380 mL | |
| 5 mM | 0.5708 mL | 2.8538 mL | 5.7076 mL | |
| 10 mM | 0.2854 mL | 1.4269 mL | 2.8538 mL |