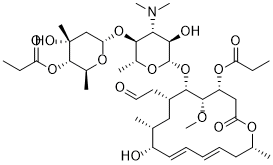
Midecamycin, a novel and potent acetoxy-substituted macrolide antibiotic, is a naturally occurring 16-membered macrolide that belongs to the acetoxy-substituted macrolide antibiotics. In this molecule, an acetoxy group is substituted on the position 9 of the 16-member ring and on position 4 of the terminal sugar.
Physicochemical Properties
| Molecular Formula | C41H67NO15 |
| Molecular Weight | 813.9684 |
| Exact Mass | 813.451 |
| Elemental Analysis | C, 60.50; H, 8.30; N, 1.72; O, 29.48 |
| CAS # | 35457-80-8 |
| Related CAS # | 55881-07-7 (acetate);35457-80-8; |
| PubChem CID | 5282169 |
| Appearance | White to off-white solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 874.0±65.0 °C at 760 mmHg |
| Melting Point | 155℃ -156℃ |
| Flash Point | 482.4±34.3 °C |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.536 |
| LogP | 3.53 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 14 |
| Heavy Atom Count | 57 |
| Complexity | 1360 |
| Defined Atom Stereocenter Count | 16 |
| SMILES | O([C@]1([H])C([H])([H])C(C([H])([H])[H])([C@]([H])(C([H])(C([H])([H])[H])O1)OC(C([H])([H])C([H])([H])[H])=O)O[H])[C@]1([H])C([H])(C([H])([H])[H])O[C@]([H])(C([H])(C1([H])N(C([H])([H])[H])C([H])([H])[H])O[H])O[C@]1([H])[C@]([H])([C@@]([H])(C([H])([H])C(=O)O[C@]([H])(C([H])([H])[H])C([H])([H])C([H])=C([H])C([H])=C([H])[C@@]([H])([C@]([H])(C([H])([H])[H])C([H])([H])[C@]1([H])C([H])([H])C([H])=O)O[H])OC(C([H])([H])C([H])([H])[H])=O)OC([H])([H])[H] |c:83,87| |
| InChi Key | DMUAPQTXSSNEDD-QALJCMCCSA-N |
| InChi Code | InChI=1S/C41H67NO15/c1-11-30(45)54-29-21-32(47)51-24(4)16-14-13-15-17-28(44)23(3)20-27(18-19-43)37(38(29)50-10)57-40-35(48)34(42(8)9)36(25(5)53-40)56-33-22-41(7,49)39(26(6)52-33)55-31(46)12-2/h13-15,17,19,23-29,33-40,44,48-49H,11-12,16,18,20-22H2,1-10H3/b14-13+,17-15+/t23-,24-,25-,26+,27+,28+,29-,33+,34-,35-,36-,37+,38+,39+,40+,41-/m1/s1 |
| Chemical Name | 7-(Formylmethyl)-4,10-dihydroxy-5-methoxy-9,16-dimethyl-2-oxooxacyclohexadeca-11,13-dien-6-yl 3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-alpha-L-ribo-hexopyranosyl)-3-(dimethylamino)-beta-D-glucopyranoside 4',4''-dipropionate (ester) |
| Synonyms | SF 837; SF-837; SF837; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Macrolide |
| ln Vitro | Most strains of Haemophilus, Listeria, and staphylococci are inhibited by midecamycin at concentrations below 3.1 μg/mL[1]. Midecamycin is a macrolide with 16 members. A novel macrolide antibiotic called midecamycin is made by Streptomyces mycarofaciens[2]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Midecamycin is rapidly and almost completely absorbed when orally administered. It is mainly absorbed in the alkaline intestinal environment. This rapid absorption is due to its liposoluble property which allows for good penetration in the tissues, especially bronchial secretion, prostatic tissue, middle ear exudates and bone tissue. The tissue/serum ratio concentration is greater than 1 which indicates that this product does not stay long in the plasma. After oral administration of 600 mg of midecamycin, the peak serum concentration is 0.8 mg/L and it is attained 1 hour after oral administration. This concentration dereased significantly after 4-6 hours. The major route of elimination of midecamycin is is the liver, followed by a low significance of renal elimination. Urinary concentrations accounts for about 3.3% of the administered dose after 6 hours. The reported apparent volume of distribution of midecamycin is 7.7 L/kg. Midecamycin presentas a low renal clearance value. Metabolism / Metabolites Midecamycin undergoes extensive biotransformation in the liver and its metabolites are characterized by presenting little to no antimicrobial activity. The main metabolite is formed by a 14-hydroxylation and it can be also detected in urine. Biological Half-Life The half-life of midecamycin is longer than the first macrolide antibiotics. after intravenous administration, the half-life reported is of 54 minutes. |
| Toxicity/Toxicokinetics |
Protein Binding Midecamycin does not bind to plasma proteins in a significant proportion and thus, the bound form can account for about 15% of the administered dose. The acetate form of midecamycin presents a larger protein binding. |
| References |
[1]. Neu HC. In vitro activity of midecamycin, a new macrolide antibiotic. Antimicrob Agents Chemother. 1983 Sep;24(3):443-4. [2]. Cloning and characterization of genes encoded in dTDP-D-mycaminose biosynthetic pathway from amidecamycin-producing strain, Streptomyces mycarofaciens. Acta Biochim Biophys Sin (Shanghai). 2007 Mar;39(3):187-93. |
| Additional Infomation |
Midecamycin is an organic molecular entity. Midecamycin is a naturally occurring 16-membered macrolide that fits under the category of acetoxy-substituted macrolide antibiotics. In this molecule, an acetoxy group is substituted on the position 9 of the 16-member ring and on position 4 of the terminal sugar. Until 2017, midecamycin was still under the list of approved antimicrobial active pharmaceutical ingredients by Health Canada. Drug Indication Midecamycin was used for the treatment of infections in the oral cavity, upper and lower respiratory tracts and skin and soft tissue infections. The alone use of midecamycin was mainly used in Europe or Japan. Mechanism of Action Midecamycin, as part of the macrolides, act by inhibiting bacterial protein synthesis. More specifically, midecamycin inhibits bacterial growth by targetting the 50S ribosomal subunit preventing peptide bond formation and translocation during protein synthesis. The presence of mutations in the 50S RNA can prevent midecamycin binding. Midecamycin is a broad spectrum antibiotic and thus, it can interact with different bacteria. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 36~100 mg/mL ( 44.23~122.85 mM ) Ethanol : ~100 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.25 mg/mL (2.76 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.25 mg/mL (2.76 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.25 mg/mL (2.76 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.25 mg/mL (2.76 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2285 mL | 6.1427 mL | 12.2855 mL | |
| 5 mM | 0.2457 mL | 1.2285 mL | 2.4571 mL | |
| 10 mM | 0.1229 mL | 0.6143 mL | 1.2285 mL |