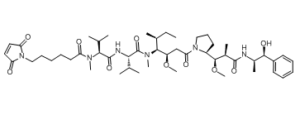
Mc-MMAE is a novel protective group for MMAE [(maleimidocaproyl)-conjugated monomethyl auristatin E], which is a highly potent mitotic/tubulin inhibitor, and is a toxin payload/warhead in antibody drug conjugate (ADC). Effective antibody-drug conjugates (ADC) combine efficient intratumoral drug release with high drug-linker stability in circulation. Monomethyl auristatin E (MMAE) was conjugated to the anti-CD30 monoclonal antibody (mAb), cAC10, to generate a potent and selective ADC against models of Hodgkin's disease and CD30(+) anaplastic large cell lymphoma.
Physicochemical Properties
| Molecular Formula | C49H78N6O10 |
| Molecular Weight | 911.17782 |
| Exact Mass | 910.578 |
| Elemental Analysis | C, 64.59; H, 8.63; N, 9.22; O, 17.56 |
| CAS # | 863971-24-8 |
| Related CAS # | 863971-24-8 |
| PubChem CID | 59168780 |
| Appearance | White to light yellow solid |
| Density | 1.144±0.06 g/cm3(Predicted) |
| Boiling Point | 1025.2±65.0 °C(Predicted) |
| LogP | 4.911 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 26 |
| Heavy Atom Count | 65 |
| Complexity | 1620 |
| Defined Atom Stereocenter Count | 10 |
| SMILES | [C@@H]([C@@H]1CCCN1C(=O)C[C@@H](OC)[C@H]([C@@H](C)CC)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](C(C)C)N(C)C(=O)CCCCCN1C(=O)C=CC1=O)(OC)[C@@H](C)C(=O)N[C@H](C)[C@H](C1C=CC=CC=1)O |
| InChi Key | UGJOTJHSQWBROP-AXJCKIDNSA-N |
| InChi Code | InChI=1S/C49H78N6O10/c1-13-32(6)44(37(64-11)29-41(59)54-28-20-23-36(54)46(65-12)33(7)47(61)50-34(8)45(60)35-21-16-14-17-22-35)53(10)49(63)42(30(2)3)51-48(62)43(31(4)5)52(9)38(56)24-18-15-19-27-55-39(57)25-26-40(55)58/h14,16-17,21-22,25-26,30-34,36-37,42-46,60H,13,15,18-20,23-24,27-29H2,1-12H3,(H,50,61)(H,51,62)/t32-,33+,34+,36-,37+,42-,43-,44-,45+,46+/m0/s1 |
| Chemical Name | 6-(2,5-dioxopyrrol-1-yl)-N-[(2S)-1-[[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]-N-methylhexanamide |
| Synonyms | Mc-MMAE; Maleimidocaproyl-monomethylauristatin E |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture.(2). This product is not stable in solution, please use freshly prepared working solution for optimal results. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Auristatin |
| ln Vitro | Maleimidocaproic acid must be added to a solution of MMAE in methylene chloride in order to synthesize Mc-MMAE. Diethyl cyanophosphate and diisoacetyl amide must then be added [1]. |
| Animal Protocol | The HRP-MMAE reporter enzyme-drug conjugate is created by thiolating horseradish peroxidase (HRP) with 2-iminothiolane and conjugating it to mc-MMAE. Briefly, a thiolation reaction mixture containing 0.2 mM HRP (8 mg/mL) and 50 mM 2-iminothiolane in 25 mM sodium borate decahydrate (Na2B4O7•10H2O) buffer (pH 9.0) is incubated for 1 hour at 37°C. The removal of unreacted 2-iminothiolane is achieved by passing it through a PD-10 desalting column that has been adjusted to pH 7.4. Peak fractions are combined, and a 3:1 molar ratio is used to couple mc-MMAE to thiolated HRP (HRP-SH). The final conjugation reaction mixture contained 80 μM HRP-SH (3.2 mg/mL) in sodium borate buffer [50 mM H3BO3, 50 mM NaCl (pH 8.0); 80% v/v] and 240 μM mc-MMAE in ice-cold CH3CN (20% v/v). After 30 minutes on ice, the reaction is terminated with a 20-fold molar excess of free cysteine (4.8 mM) before PD-10 chromatography. Peak fractions containing HRP-MMAE (exchanged into PBS) are pooled and evaluated for extent of modification using the thiol-reactive dye, Alexa Fluor 594 C5 maleimide[1]. |
| References |
[1]. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res. 2005 Jan 15;11(2 Pt 1):843-52. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ~180 mg/mL (~197.56 mM) H2O 中的溶解度: < 0.1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: 4.5 mg/mL (4.94 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 45.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 4.5 mg/mL (4.94 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 45.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0975 mL | 5.4874 mL | 10.9748 mL | |
| 5 mM | 0.2195 mL | 1.0975 mL | 2.1950 mL | |
| 10 mM | 0.1097 mL | 0.5487 mL | 1.0975 mL |