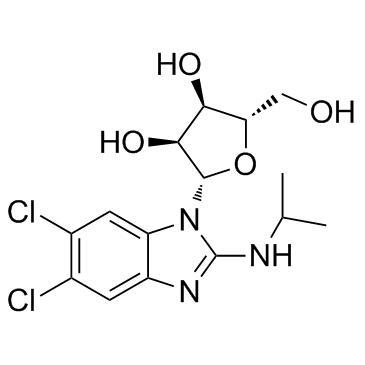
Maribavir (1263W94; BW-1263W94; GW-1263; SHP-620; GW-257406X; VP-41263; Livtencity), an experimental oral antiviral (anti-CMV) drug candidate, is a novel and potent inhibitor of histone phosphorylation catalyzed by wild-type pUL97 in vitro with an IC50 of 3 nM. As of December 2021, Maribavir has been approved as the first drug for treating adults and pediatric patients with post-transplant cytomegalovirus (CMV) infection/disease that does not respond to available antiviral treatment for CMV. Livtencity works by preventing the activity of human cytomegalovirus enzyme pUL97, thus blocking virus replication. Maribavir has potent antiviral activity against HCMV and Epstein-Barr virus (EBV). Maribavir is licensed by ViroPharma from GlaxoSmithKline in 2003 for the prevention and treatment of human cytomegalovirus (HCMV) disease in hematopoietic stem cell/bone marrow transplant patients.
Physicochemical Properties
| Molecular Formula | C15H19CL2N3O4 |
| Molecular Weight | 376.23506 |
| Exact Mass | 375.075 |
| Elemental Analysis | C, 47.89; H, 5.09; Cl, 18.84; N, 11.17; O, 17.01 |
| CAS # | 176161-24-3 |
| PubChem CID | 471161 |
| Appearance | Solid powder |
| Density | 1.7±0.1 g/cm3 |
| Boiling Point | 611.0±65.0 °C at 760 mmHg |
| Flash Point | 323.3±34.3 °C |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.703 |
| LogP | 2.71 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 24 |
| Complexity | 447 |
| Defined Atom Stereocenter Count | 4 |
| SMILES | ClC1=C(Cl)C=C2C(N=C(NC(C)C)N2[C@@H]3[C@@H](O)[C@@H](O)[C@H](CO)O3)=C1 |
| InChi Key | KJFBVJALEQWJBS-XUXIUFHCSA-N |
| InChi Code | InChI=1S/C15H19Cl2N3O4/c1-6(2)18-15-19-9-3-7(16)8(17)4-10(9)20(15)14-13(23)12(22)11(5-21)24-14/h3-4,6,11-14,21-23H,5H2,1-2H3,(H,18,19)/t11-,12-,13-,14-/m0/s1 |
| Chemical Name | 5,6-Dichloro-2-(isopropylamino)-1-beta-L-ribofuranosyl-1H-benzimidazole |
| Synonyms | 1263-W94; GW-1263; SHP-620; 1263 W94; 1263W-94; BW1263W 94; GW1263; SHP620; BW-1263W94; VP41263; Livtencity; BW 1263W94; VP-41263; GW-257406X; GW257406-X; GW 257406X; GW 257406 X; GW257406X |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HCMV |
| ln Vitro | Maribavir, with a mean IC50 of 35 nM, is a strong inhibitor of autophosporylation of both the wild type and all of the major Ganciclovir (GCV) resistant UL97 mutants that have been studied. With an IC50 of 4.8 nM, the M460I mutation causes hypersensitivity to maribavir. L397R, a UL97 mutant resistant to maribavir, exhibits reduced function as a Ganciclovir kinase and protein kinase (~10% of wild type levels). Maribavir is a competitive inhibitor of ATP with a Ki of 10 nM, as shown by enzyme kinetic studies[1]. Using a multicycle DNA hybridization assay, maribavir (1263W94) inhibits viral replication in a dose-dependent manner with an IC50 of 0.12±0.01 μM. Maribavir significantly inhibits the pUL97 protein kinase, with 50% inhibition happening at 3 nM[2]. |
| Enzyme Assay | Using increasing concentrations of ATP (2 μM to 20 μM), enzyme kinetic analysis is carried out on the purified wild type and mutant UL97 protein species. To calculate the Km for ATP for each UL97 species, the amount of incorporated radiolabelled phosphate is plotted against the concentration of ATP in a Lineweaver Burke plot. Protein kinase assays are used to measure the impact of Maribavir on the rate of radiolabelled phosphate incorporation by either wild type or mutant UL97.The assays can be conducted at a fixed concentration of 0.55 μM of Maribavir, as previously mentioned, or with increasing concentrations of 0.01 μM to 5.0 μM of Maribavir to determine the IC50 of Maribavir for each species of UL97.Plots of 1/v vs 1/ATP with increasing Maribavir concentrations are created to ascertain the type of inhibition mediated by Maribavir. If the family of lines converged at 1/Vmax on the y-axis, then there was clear evidence of competitive inhibition. The Ki is computed using the variation in slope brought about by the addition of Maribavir[1]. |
| Cell Assay | MRC-5 cells are cultivated for three days in MEM 8-1-1 until confluence (~1.1×105 cells/well), after being seeded in 24-well plates at a density of approximately 5×104 cells/well. In MEM 2-1-1, the cells are infected with AD169 at a multiplicity of infection (MOI) of 1 to 3, and the infection is allowed to adsorb for 90 minutes at 37°C. One milliliter of MEM 2-1-1 is added in place of the unadsorbed virus. Maribavir, BDCRB, or GCV are added to the medium at the concentrations specified for each experiment to examine the impact of the compounds on viral DNA synthesis or maturation[2]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Population pharmacokinetic modeling in patients receiving maribavir 400mg twice daily showed an AUC0-tau and Cmax of 128 µg.h/mL and 17.2 µg/mL, respectively. It has a median Tmax of one to three hours. Maribavir is eliminated primarily via hepatic metabolism. Following the oral administration of radiolabeled maribavir, 61% of the dose was excreted in the urine (<2% as unchanged drug) and 14% was excreted in the feces (5.7% as unchanged drug). The mean apparent steady-state volume of distribution for maribavir was 27.3 L. In post-transplant patients, the mean oral clearance of maribavir was 2.85 L/h. Metabolism / Metabolites Maribavir is extensively metabolized following oral administration, primarily by CYP3A4 and, to a lesser extent, by CYP1A2. Its major circulating metabolite is VP 44469, an inactive N-dealkylated metabolite. Biological Half-Life In post-transplant patients, the mean half-life of elimination was 4.32 hours. |
| Toxicity/Toxicokinetics |
Hepatotoxicity In large preregistration clinical trials, ALT elevations occurred in 3.5% of maribavir vs less than 1% of standard therapy in recipients with refractory CMV infection after hematopoietic cell transplantation. The ALT elevations were transient, mild and asymptomatic. In prelicensure studies, there were no instances of clinically apparent liver injury with jaundice. Since the approval of maribavir and its general availability, there have been no reported cases of clinically apparent liver injury with jaundice associated with its use; however, the total clinical experience with maribavir therapy is limited. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Across all concentration ranges tested, maribavir was extensively (~98%) protein-bound in plasma, likely primarily to serum albumin and alpha-1-acid glycoprotein. |
| References |
[1]. The effects of Maribavir on the autophosphorylation of ganciclovir resistant mutants of the cytomegalovirus UL97 protein. Herpesviridae. 2010 Dec 7;1(1):4. [2]. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002 Aug;46(8):2365-72. |
| Additional Infomation |
Maribavir is an inhibitor of the cytomegalovirus (CMV; HHV5) pUL97 kinase which is used to treat CMV infections in patients post-transplantation. Most standard CMV therapies, such as [ganciclovir] or [foscarnet], target CMV DNA polymerase - while generally effective, these medications tend to promote the development of CMV resistance to DNA polymerase-based therapies, and their use is often limited by toxicities like myelosuppression and renal injury. Maribavir is novel in that it instead targets the CMV pUL97 kinase, thereby providing an effective alternative treatment option in cases of resistant infections. Maribavir was approved by the FDA in November 2021, under the name Livtencity (Takeda), for the treatment of resistant CMV infections in post-transplant patients. The drug was also approved by Health Canada in September 2022 and by European Commission in November 2022. Maribavir is a Cytomegalovirus pUL97 Kinase Inhibitor. The mechanism of action of maribavir is as a Cytomegalovirus pUL97 Kinase Inhibitor, and Cytochrome P450 3A4 Inhibitor, and P-Glycoprotein Inhibitor, and Breast Cancer Resistance Protein Inhibitor. Maribavir is an orally available, antiviral agent which inhibits the pUL97 kinase of cytomegalovirus (CMV) and is used to treat refractory forms of post-transplant CMV infection. Maribavir has been associated with low rates of mild-to-moderate serum aminotransferase elevations during therapy but has not been linked to cases of clinically apparent acute liver injury. Maribavir is an orally available benzimidazole riboside compound with activity against cytomegalovirus (CMV). Maribavir is a selective ATP competitor of viral UL97 kinase, which is involved in viral nuclear maturation events, such as viral DNA assembly and movement of viral capsids from the nucleus of infected cells. Maribavir has activity against strains of CMV that are resistant to standard anti-CMV agents. Drug Indication Maribavir is indicated for the treatment of post-transplant cytomegalovirus (CMV) infection (following hematopoietic stem cell transplant or solid organ transplant) which is refractory to standard treatment with [ganciclovir], [valganciclovir], [cidofovir], or [foscarnet]. In the US, patients receiving the treatment should weigh more than 35 kg and be at least 12 years old. In Canada and Europe, maribavir is only approved in adults. LIVTENCITY is indicated for the treatment of cytomegalovirus (CMV) infection and/or disease that are refractory (with or without resistance) to one or more prior therapies, including ganciclovir, valganciclovir, cidofovir or foscarnet in adult patients who have undergone a haematopoietic stem cell transplant (HSCT) or solid organ transplant (SOT). Consideration should be given to official guidance on the appropriate use of antiviral agents. Treatment of cytomegalovirus (CMV) infection Cytomegaloviral disease Mechanism of Action Human cytomegalovirus (CMV) is a herpesvirus commonly causing infection in patients following stem cell or organ transplants. As with other herpesviruses, CMV tends to persist in the host and become reactivated under immunosuppressive conditions - patients requiring multiple immunosuppressive medications to combat transplant rejection are thus at a much higher risk of developing serious CMV infections. Maribavir belongs to a class of anti-cytomegalovirus antivirals called benzimidazole ribosides. It competitively inhibits the human CMV pUL97 viral protein kinase, which results in viable but severely defective viruses upon replication, although the reasons for this remain poorly defined. In addition, maribavir also inhibits viral release from the nucleus to the cytoplasm by inhibiting pUL97-dependent phosphorylation of the nuclear lamina component lamin A/C, although the extent to which this activity contributes to its antiviral efficacy is unclear. Pharmacodynamics Maribavir exerts its antiviral efficacy via an alternative target as compared to traditional CMV antivirals and is thus useful in the treatment of CMV infections that have proven resistant to standard therapy. Maribavir should not be used concomitantly with ganciclovir or valganciclovir, as these molecules both require activation via CMV pUL97 in order to exert their antiviral effect. Taking them alongside maribavir - an inhibitor of this same kinase - will therefore significantly reduce their antiviral activity. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~200 mg/mL (~531.58 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.87 mg/mL (7.63 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.87 mg/mL (7.63 mM) (saturation unknown) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.64 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 4: ≥ 2.5 mg/mL (6.64 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 5: ≥ 2.5 mg/mL (6.64 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 6: 5% DMSO+40% PEG300+5% Tween-80+50% Saline: ≥ 2.87 mg/mL (7.63 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6579 mL | 13.2894 mL | 26.5788 mL | |
| 5 mM | 0.5316 mL | 2.6579 mL | 5.3158 mL | |
| 10 mM | 0.2658 mL | 1.3289 mL | 2.6579 mL |