Physicochemical Properties
| Molecular Formula | C57H90N12O20 |
| Molecular Weight | 1263.39291524887 |
| Exact Mass | 1262.639 |
| CAS # | 2254483-96-8 |
| PubChem CID | 132282519 |
| Appearance | Typically exists as solid at room temperature |
| LogP | -2 |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 21 |
| Rotatable Bond Count | 23 |
| Heavy Atom Count | 89 |
| Complexity | 2650 |
| Defined Atom Stereocenter Count | 10 |
| SMILES | CCC(CC/C=C\C=C\C(NC(C(NC1C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NC(C(C(=O)O)O)C(=O)NC(CC(=O)O)C(=O)NCC(=O)NC(C(C(=O)O)C)C(=O)NC(C(C)C)C(=O)N2C(CC(C2)C)C(=O)NC1C)=O)C(C(=O)O)C)=O)C |
| InChi Key | SMTCDPMWINUUKC-AVBBYWJWSA-N |
| InChi Code | InChI=1S/C57H90N12O20/c1-11-28(6)18-14-12-13-15-20-36(70)63-41(30(8)55(84)85)51(80)67-43-32(10)60-48(77)35-22-29(7)25-69(35)54(83)40(27(4)5)66-50(79)42(31(9)56(86)87)64-37(71)24-59-46(75)34(23-38(72)73)62-53(82)44(45(74)57(88)89)68-47(76)33(19-16-17-21-58)61-49(78)39(26(2)3)65-52(43)81/h12-13,15,20,26-35,39-45,74H,11,14,16-19,21-25,58H2,1-10H3,(H,59,75)(H,60,77)(H,61,78)(H,62,82)(H,63,70)(H,64,71)(H,65,81)(H,66,79)(H,67,80)(H,68,76)(H,72,73)(H,84,85)(H,86,87)(H,88,89)/b13-12-,20-15+/t28?,29-,30?,31?,32?,33+,34+,35+,39-,40+,41+,42-,43+,44+,45?/m1/s1 |
| Chemical Name | (3S)-4-[[(3S,6R,12S,15S,18S,21R,24S,28S,30R)-18-(4-aminobutyl)-6-(1-carboxyethyl)-15-[carboxy(hydroxy)methyl]-12-(carboxymethyl)-25,30-dimethyl-2,5,8,11,14,17,20,23,27-nonaoxo-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,26-nonazabicyclo[26.3.0]hentriacontan-24-yl]amino]-2-methyl-3-[[(2E,4Z)-8-methyldeca-2,4-dienoyl]amino]-4-oxobutanoic acid |
| Synonyms | Malacidin B; CHEBI:140174; (3S)-4-[[(3S,6R,12S,15S,18S,21R,24S,28S,30R)-18-(4-aminobutyl)-6-(1-carboxyethyl)-15-[carboxy(hydroxy)methyl]-12-(carboxymethyl)-25,30-dimethyl-2,5,8,11,14,17,20,23,27-nonaoxo-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,26-nonazabicyclo[26.3.0]hentriacontan-24-yl]amino]-2-methyl-3-[[(2E,4Z)-8-methyldeca-2,4-dienoyl]amino]-4-oxobutanoic acid; 2254483-96-8; N-[(2S)-3-amino-2-({3-methyl-N-[(2E,4Z)-8-methyldeca-2,4-dienoyl]-L-alpha-aspartyl}amino)butanoyl]-D-valyl-L-lysyl-3-hydroxy-L-alpha-aspartyl-L-alpha-aspartylglycyl-3-methyl-D-alpha-aspartyl-L-valyl-(4R)-4-methyl-L-proline; (3S)-4-(((3S,6R,12S,15S,18S,21R,24S,28S,30R)-18-(4-aminobutyl)-6-(1-carboxyethyl)-15-(carboxy(hydroxy)methyl)-12-(carboxymethyl)-25,30-dimethyl-2,5,8,11,14,17,20,23,27-nonaoxo-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,26-nonazabicyclo(26.3.0)hentriacontan-24-yl)amino)-2-methyl-3-(((2E,4Z)-8-methyldeca-2,4-dienoyl)amino)-4-oxobutanoic acid; N-((2S)-3-amino-2-((3-methyl-N-((2E,4Z)-8-methyldeca-2,4-dienoyl)-L-alpha-aspartyl)amino)butanoyl)-D-valyl-L-lysyl-3-hydroxy-L-alpha-aspartyl-L-alpha-aspartylglycyl-3-methyl-D-alpha-aspartyl-L-valyl-(4R)-4-methyl-L-proline; CHEMBL4470512; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Lipopeptide |
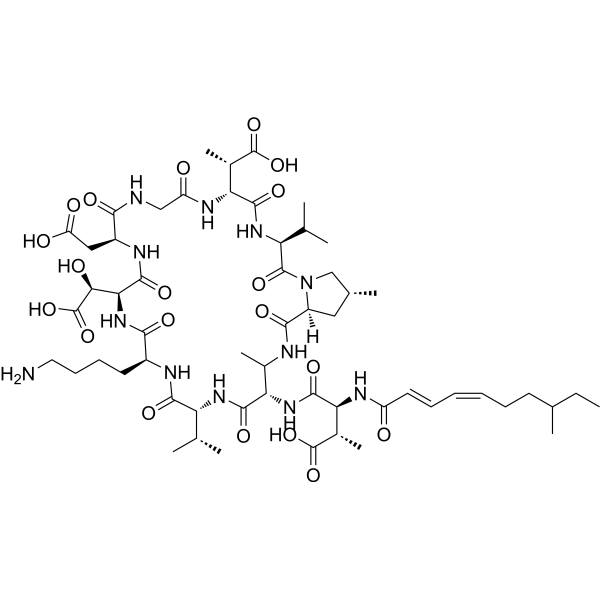
| ln Vitro | As mentioned before, it is assumed that less than 1% of all environmental microbes have been cultured in laboratory settings as a source to discover new antimicrobial molecules. Through applying emerging technologies such as environment-mimicking cultivation and genome mining of Biosynthetic Gene Clusters (BGCs), Brady and colleagues have discovered malacidin A and malacidin B (8a, 8b; Figure 2) as members of a new class of calcium-dependent macrocyclic lipopeptide antibiotics. Malacidin A consists of a macrocyclic nonapeptide, containing four nonproteinogenic amino acids, and an unsaturated C9-fatty chain acylated to an exocyclic β-methylaspartic acid. The total synthesis of malacidin A has been reported by Sun et al. and the absolute configurations of its five nonproteinogenic amino acid residues have been elucidated. Malacidin A exhibits broad activity against Gram-positive bacteria including methicillin-resistant S. aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) and has potent antimicrobial activity (MIC range 0.2–2 μg/mL) in the presence of Ca2+ ions. Malacidin A was also able to clear S. aureus infection in a skin infection rat model. The MoA of malacidins involves binding to lipid II as described for other known antibiotics like vancomycin and teixobactin. However, since no cross resistance to vancomycin has been observed, binding of malacidin A to lipid II must be different from the binding mode of vancomycin. [1] |
| References |
[1]. Emerging peptide antibiotics with therapeutic potential. Med Drug Discov. 2021 Mar;9:100078. [2]. Lakemeyer M, Zhao W, Mandl FA, Hammann P, Sieber SA. Thinking Outside the Box-Novel Antibacterials To Tackle the Resistance Crisis. Angew Chem Int Ed Engl. 2018 Oct 26;57(44):14440-14475. |
| Additional Infomation |
Malacidin B is a homodetic cyclic peptide that is malacidin A in which the (2E,4Z)-8-methylnona-2,4-dienoyl group has been replaced by a (2E,4Z)-8-methyldeca-2,4-dienoyl group. It has a role as a bacterial metabolite, a member of calcium-dependent antibiotics and an antibacterial agent. It is a homodetic cyclic peptide and a lipopeptide. Malacidin B, along with [DB14051], is a member of a class of chemicals made by bacteria found in soil that can kill Gram-positive bacteria. Malacidins are 10-member macrocycle lipopeptides discovered via gene sequencing and bioinformatic analysis. While structurally similar to other macrocycle drugs like [DB00080] and [DB06087], Malacidin B appears to act via its own distinct mechanism. Drug Indication Malacidin B is being investigated for its antibiotic action and has potential for use as an antibacterial agent in the future. Mechanism of Action Malacidin B appears to bind Lipid II via a calcium dependent mechanism despite the absence of the typical Asp-X-Asp-Gly motif associate with calcium binding. The structure of Malacidin B includes a 3-hydroxy-aspartate residue while the "X" variable spacer residue is absent. It is unknown how these unique structural features may impact the drug's mechanism of action. The binding of Malacidin B to Lipid II prevents the incorporation of the subunit into the cell wall, disrupting synthesis and likely resulting in death of the bacterial cell. Malacidin B does not appear to form pores nor does it seem to integrate into the cell wall. While this mechanism is similar to that of [DB00512], Malacidin B retains its activity against [DB00512]-resistant pathogens. Unlike other antibiotic agents, Malacidin B also retains its activity in the presence of pulmonary surfactants. Pharmacodynamics Malacidin B disrupts bacterial cell wall synthesis likely leading to cell death in Gram-positive bacteria. This bactericidal effect reduces the number of live bacteria present during infection. Malacidin B has exhibited broad spectrum activity against Gram-positive bacteria including several multi-drug resistant pathogens. This review covers some of the recent progress in the field of peptide antibiotics with a focus on compounds with novel or established mode of action and with demonstrated efficacy in animal infection models. Novel drug discovery approaches, linear and macrocyclic peptide antibiotics, lipopeptides like the polymyxins as well as peptides addressing targets located in the plasma membrane or in the outer membrane of bacterial cells are discussed.[1] The public view on antibiotics as reliable medicines changed when reports about "resistant superbugs" appeared in the news. While reasons for this resistance development are easily spotted, solutions for re-establishing effective antibiotics are still in their infancy. This Review encompasses several aspects of the antibiotic development pipeline from very early strategies to mature drugs. An interdisciplinary overview is given of methods suitable for mining novel antibiotics and strategies discussed to unravel their modes of action. Select examples of antibiotics recently identified by using these platforms not only illustrate the efficiency of these measures, but also highlight promising clinical candidates with therapeutic potential. Furthermore, the concept of molecules that disarm pathogens by addressing gatekeepers of virulence will be covered. The Review concludes with an evaluation of antibacterials currently in clinical development. Overall, this Review aims to connect select innovative antimicrobial approaches to stimulate interdisciplinary partnerships between chemists from academia and industry.[2] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7915 mL | 3.9576 mL | 7.9152 mL | |
| 5 mM | 0.1583 mL | 0.7915 mL | 1.5830 mL | |
| 10 mM | 0.0792 mL | 0.3958 mL | 0.7915 mL |