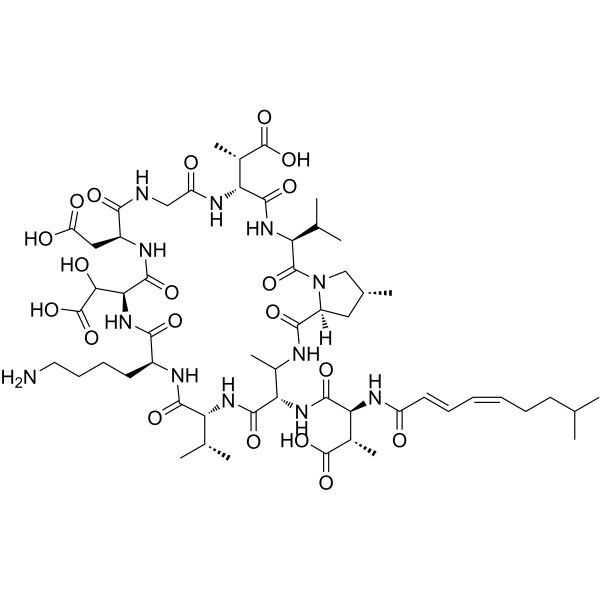
Physicochemical Properties
| Molecular Formula | C56H88N12O20 |
| Molecular Weight | 1249.36633491516 |
| Exact Mass | 1248.623 |
| CAS # | 2254483-95-7 |
| PubChem CID | 132282518 |
| Appearance | Typically exists as solid at room temperature |
| LogP | -2.5 |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 21 |
| Rotatable Bond Count | 22 |
| Heavy Atom Count | 88 |
| Complexity | 2630 |
| Defined Atom Stereocenter Count | 10 |
| SMILES | CC(CC/C=C\C=C\C(NC(C(NC1C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NC(C(C(=O)O)O)C(=O)NC(CC(=O)O)C(=O)NCC(=O)NC(C(C(=O)O)C)C(=O)NC(C(C)C)C(=O)N2C(CC(C2)C)C(=O)NC1C)=O)C(C(=O)O)C)=O)C |
| InChi Key | USNOUEMKNKRWQT-ORRWAHHJSA-N |
| InChi Code | InChI=1S/C56H88N12O20/c1-25(2)17-13-11-12-14-19-35(69)62-40(29(8)54(83)84)50(79)66-42-31(10)59-47(76)34-21-28(7)24-68(34)53(82)39(27(5)6)65-49(78)41(30(9)55(85)86)63-36(70)23-58-45(74)33(22-37(71)72)61-52(81)43(44(73)56(87)88)67-46(75)32(18-15-16-20-57)60-48(77)38(26(3)4)64-51(42)80/h11-12,14,19,25-34,38-44,73H,13,15-18,20-24,57H2,1-10H3,(H,58,74)(H,59,76)(H,60,77)(H,61,81)(H,62,69)(H,63,70)(H,64,80)(H,65,78)(H,66,79)(H,67,75)(H,71,72)(H,83,84)(H,85,86)(H,87,88)/b12-11-,19-14+/t28-,29?,30?,31?,32+,33+,34+,38-,39+,40+,41-,42+,43+,44?/m1/s1 |
| Chemical Name | (3S)-4-[[(3S,6R,12S,15S,18S,21R,24S,28S,30R)-18-(4-aminobutyl)-6-(1-carboxyethyl)-15-[carboxy(hydroxy)methyl]-12-(carboxymethyl)-25,30-dimethyl-2,5,8,11,14,17,20,23,27-nonaoxo-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,26-nonazabicyclo[26.3.0]hentriacontan-24-yl]amino]-2-methyl-3-[[(2E,4Z)-8-methylnona-2,4-dienoyl]amino]-4-oxobutanoic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Malacidin A has calcium-dependent antibacterial action against methicillin-resistant Staphylococcus aureus (MRSA)[1]. With MIC values ranging from 0.1 to 2.0 µg/mL, malacidin A (100-250 µg/mL) has broad activity against Gram-positive bacteria, including pathogens resistant to multiple drugs and microorganisms resistant to mechanistically different, clinically utilized antibiotics[1]. |
| ln Vivo | In mice with cutaneous wound infection, malacidin A (4 mg/kg) has antibacterial activity and reduces the amount of bacteria present in the wound[1]. |
| Animal Protocol |
Animal/Disease Models: Male Sprague Dawley rats (8 weeks old) with cutaneous wound infection model[1] Doses: 4 mg/kg Route of Administration: Apply over the wound Experimental Results: Had no observed bacterial burdens in the wounds. |
| References |
[1]. Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Nat Microbiol. 2018 Apr;3(4):415-422. [2]. A Concise Synthetic Strategy Towards the Novel Calcium-dependent Lipopeptide Antibiotic, Malacidin A and Analogues. Front Chem. 2021 Aug 4;9:687875. |
| Additional Infomation |
Malacidin A is a homodetic cyclic peptide containing a 28-membered ring and consisting of 3-methyl-N-[(2E,4Z)-8-methylnona-2,4-dienoyl]-L-alpha-aspartyl, (2S)-2,3-diaminobutanoyl, D-valyl, L-lysyl, 3-hydroxy-L-alpha-aspartyl, L-alpha-aspartyl, glycyl, 3-methyl-D-alpha-aspartyl, L-valyl, and (4R)-methyl-L-proline joined in sequence and cyclised by condensation of the beta-amino group of the 2,3-diaminobutanoyl residue with the carboxy group of the 4-methylproline residue. It has a role as a bacterial metabolite, a member of calcium-dependent antibiotics and an antibacterial agent. It is a homodetic cyclic peptide and a lipopeptide. Malacidin A, along with [DB14052], is a member of a class of chemicals made by bacteria found in soil that can kill Gram-positive bacteria. Malacidins are 10-member macrocycle lipopeptides discovered via gene sequencing and bioinformatic analysis. While structurally similar to other macrocycle drugs like [DB00080] and [DB06087], Malacidin A appears to act via its own distinct mechanism. Drug Indication Malacidin A is being investigated for its antibiotic action and has potential for use as an antibacterial agent in the future. Mechanism of Action Malacidin A appears to bind Lipid II via a calcium dependent mechanism despite the absence of the typical Asp-X-Asp-Gly motif associate with calcium binding. The structure of Malacidin A includes a 3-hydroxy-aspartate residue while the "X" variable spacer residue is absent. It is unknown how these unique structural features may impact the drug's mechanism of action. The binding of Malacidin A to Lipid II prevents the incorporation of the subunit into the cell wall, disrupting synthesis and likely resulting in death of the bacterial cell. Malacidin A does not appear to form pores nor does it seem to integrate into the cell wall. While this mechanism is similar to that of [DB00512], Malacidin A retains its activity against [DB00512]-resistant pathogens. Unlike other antibiotic agents, Malacidin A also retains its activity in the presence of pulmonary surfactants. Pharmacodynamics Malacidin A disrupts bacterial cell wall synthesis likely leading to cell death in Gram-positive bacteria. This bactericidal effect reduces the number of live bacteria present during infection. Malacidin A has exhibited broad spectrum activity against Gram-positive bacteria including several multi-drug resistant pathogens. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.8004 mL | 4.0020 mL | 8.0040 mL | |
| 5 mM | 0.1601 mL | 0.8004 mL | 1.6008 mL | |
| 10 mM | 0.0800 mL | 0.4002 mL | 0.8004 mL |