MRTX-1133 (MRTX1133; MRTX 1133) is a novel, potent, non-covalent, and selective KRAS G12D inhibitor with antitumor activity. It works in a KRASG12D mutant mouse tumor xenograft model and is currently in clinical trial for treating solid tumors. The most prevalent oncogenic KRAS mutation, KRASG12D, is a promising target for the treatment of solid tumors. However, compared to KRASG12C, selective inhibition of KRASG12D poses a significant challenge because inhibitors must bind KRASG12D with a high enough affinity to eliminate the need for covalent interactions with the mutant KRAS protein. Through extensive structure-based drug design, MRTX1133 was identified as a noncovalent, potent, and selective inhibitor of KRASG12D. MRTX1133 suppresses KRASG12D signaling in cells and in vivo, and its antitumor benefit was demonstrated in a murine animal model. To the best of our knowledge, this is the first report in the literature of a small molecule inhibitor of KRASG12D that exhibits robust in vivo efficacy. These data support the potential for the advancement of an effective therapeutic against this “undruggable” target. The optimization process was facilitated by high-resolution X-ray crystal structures. In-depth binding mode analysis derived from cocrystal structures allowed the optimization of lipophilic contact of the inhibitor in the binding pocket and the identification of nonclassical hydrogen bonding and ion pair interactions, ultimately increasing selective binding affinity for KRASG12D by more than 1,000,000-fold relative to the initial hit 5B. MRTX1133 binds to the switch II pocket and inhibits the protein–protein interactions necessary for activation of the KRAS pathway. MRTX1133 not only possesses single-digit nM potency in a cellular proliferation assay, but also demonstrates tumor regressions in the Panc 04.03 xenograft model. A more comprehensive in vitro and in vivo pharmacological characterization of MRTX1133 will be disclosed in due course.
Physicochemical Properties
| Molecular Formula | C33H31F3N6O2 |
| Molecular Weight | 600.6335 |
| Exact Mass | 600.246 |
| Elemental Analysis | C, 65.99; H, 5.20; F, 9.49; N, 13.99; O, 5.33 |
| CAS # | 2621928-55-8 |
| Related CAS # | 2621928-55-8; |
| PubChem CID | 156124857 |
| Appearance | Yellow to brown solid powder |
| LogP | 5.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 44 |
| Complexity | 1100 |
| Defined Atom Stereocenter Count | 2 |
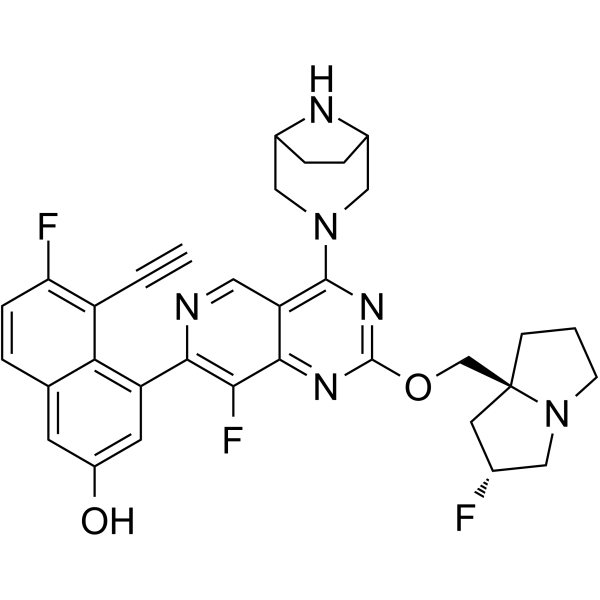
| SMILES | C#CC1=C(C=CC2=CC(=CC(=C21)C3=NC=C4C(=C3F)N=C(N=C4N5CC6CCC(C5)N6)OC[C@@]78CCCN7C[C@@H](C8)F)O)F |
| InChi Key | SCLLZBIBSFTLIN-IFMUVJFISA-N |
| InChi Code | InChI=1S/C33H31F3N6O2/c1-2-23-26(35)7-4-18-10-22(43)11-24(27(18)23)29-28(36)30-25(13-37-29)31(41-15-20-5-6-21(16-41)38-20)40-32(39-30)44-17-33-8-3-9-42(33)14-19(34)12-33/h1,4,7,10-11,13,19-21,38,43H,3,5-6,8-9,12,14-17H2/t19-,20?,21?,33+/m1/s1 |
| Chemical Name | 4-[4-(3,8-diazabicyclo[3.2.1]octan-3-yl)-8-fluoro-2-[[(2R,8S)-2-fluoro-1,2,3,5,6,7-hexahydropyrrolizin-8-yl]methoxy]pyrido[4,3-d]pyrimidin-7-yl]-5-ethynyl-6-fluoronaphthalen-2-ol |
| Synonyms | MRTX1133; MRTX 1133; MRTX-1133 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | KRas G12D (Kd = 0.2 pM) |
| ln Vitro | MRTX1133 can reversibly bind to both activated and inactivated KRAS G12D mutants and inhibit their activity. It is a highly selective inhibitor of mutant KRAS. MRTX1133 is more specific to KRAS G12D than wild-type KRAS by a factor of more than 1000. MRTX1133 not only exhibits tumor regressions in the Panc 04.03 xenograft model, but also exhibits single-digit nM potency in a cellular proliferation assay. |
| ln Vivo | MRTX1133 was discovered through an extensive structure-based activity improvement and shown to be efficacious in a KRASG12D mutant xenograft mouse tumor model. Intraperitoneal (IP) administration of MRTX1133 at 30 mg/kg in CD-1 mice resulted in sustained plasma exposure exceeding the free-fraction-adjusted pERK IC50 value in the KRASG12D mutant Panc 04.03 cell line for approximately 8 h. Encouraged by this result, we evaluated the ability to modulate KRAS-dependent ERK phosphorylation in the Panc 04.03 xenograft tumor model at 30 mg/kg BID (IP) and observed 62% and 74% inhibition of pERK signal at 1 and 12 h after the second dose, respectively. An antitumor efficacy study in this model resulted in MRTX1133 dose-dependent antitumor activity with 94% growth inhibition observed at 3 mg/kg BID (IP) and tumor regressions of −62% and −73% observed at 10 and 30 mg/kg BID (IP), respectively). In contrast, no significant antitumor activity was observed in the non-KRASG12D tumor model MKN1 (data not shown). [2]. |
| Enzyme Assay |
HTRF Binding Assay A recombinant human KRAS 4B G12D protein (corresponding to amino acids 1-169, expressed in E. Coli with C-terminal Avi biotinylated tag MW=22 kDa) was incubated with the tested compound in buffer (50 mM HEPES pH 7.5, 5 mM MgCl2, 1 mM DTT, ~0.1% DMSO), 5 nM KRASG12D, 100 nM Tracer (compound 45) and 0.5 nM Tb-SA (Cisbio). After a 1-hour incubation at room temperature, the HTRF signal was measured with a Clariostar reader [(BMG) excitation filter (Ex Tr), dichroic filter (LP TP) and emission filters (F 665-10 and F 620-10)] according to the manufacturer’s instructions. The HTRF ratio was calculated using the formula: [emission 665/emission 620] * 10000. IC50’s were fit using Xlfit software (IDBS) with the Hill equation fixed to 1 (fit Background + Bmax/(1 + ((x/IC50)^Hill))). |
| Cell Assay |
Protocol for AGS ICW Assay [2] 1. The KRASG12D mutant, AGS cells (ATCC CRL-1739), was grown in DMEM medium supplemented with 10% fetal bovine serum and Penicillin/Streptomycin. Cells were plated in black clear bottom tissue culture treated 96 well plates at a density of 20,000 cells/well and allowed to attach for 12-14 hours. The plated cells were treated with a 3-fold 9-point serial dilution of the compounds, with a top final concentration of 10 µM. The diluted compounds were added to plated cells at a final concentration of 0.5% DMSO. After 3 hours of drug treatment, the cells were fixed by incubating the plates in 50 µl of 4.0% formaldehyde at room temperature for 20 minutes. The formaldehyde was then dumped out, and 150µL of ice cold 100% methanol was added for 10 minutes to permeabilize the cells. Methanol was dumped out, and 100 µL of Licor blocking buffer (Li-Cor Biotechnology, Lincoln NE) was added for 1 hour at room temperature to inhibit non-specific antibody binding in the plates. 2. The amount of phospho-ERK was determined using an antibody specific for the phosphorylated form of ERK and compared to the amount of GAPDH. Primary antibodies used for the detection were added as follows: Phospho-ERK (Cell Signaling CS-9101) diluted 1:500 and GAPDH (Millipore MAB374) diluted 1:5000 in Odyssey blocking buffer + 0.05%Tween 20. The plates were incubated overnight at 4 °C. The plates were washed 3X with 150uL PBS + 0.1% Tween 20. 3. Secondary antibodies used to visualize primary antibodies were added as follows: Goat Anti-Rabbit-800 (LI-COR, 926-32211) and Goat Anti-Mouse680 (LI-COR, 926-68070) diluted 1:800 both in Odyssey blocking buffer +0.05% Tween20, and were incubated for 1 hour at room temperature. The plates were washed 3X with 150uL PBS +0.1% Tween20. Plates were imaged dry on a LiCOR Odyssey CLX plate reader. S192 4. The plates were analyzed by normalizing the phospho-ERK (Thr202/Tyr204) signal to the GAPDH signal for each well and percent of DMSO control values were calculated. IC50 values were generated using a 4-parameter fit of the dose response curve. Protocol for 2D Cell Proliferation Assay[2] 1. The KRASG12D mutant cell line, GP2d (Sigma-Aldrich #95090714), was grown in DMEM medium supplemented with 10% fetal bovine serum and Penicillin/Streptomycin. The KRASWT cell line, MKN1 (JCRB0252), was grown in RPMI Cells supplemented with 10% fetal bovine serum, 10mM HEPES, 10mM Sodium Pyruvate, and Penicillin/Streptomycin. Cells were plated in white clear bottom tissue culture treated 96 well plates at a density of 2,000 cells/well and allowed to attach for 12-14 hours. Each cell line was also plated in 3 wells in a baseline plate at the same density to determine the luminescence RLU values of each cell line before the drug treatment. The baseline plate was read immediately before treating plated cells with drug by incubating each of the three plated wells per cell line with 30 µL of CTG reagents for 30 mins, covered from light and shaking vigorously. The luminescence RLU values were then read on the CLARIOstar microplate reader. The plated cells were treated a 3-fold 9-point serial dilution dose response of MRTX1133, with a top final concentration of 3µM. Diluted compounds were added in a final concentration of 0.5% DMSO. After 3 days of drug treatment, each plate was read on the CLARIOstar using the conditions for the baseline plate described above. 2. The data was analyzed by subtracting the baseline RLU values from the RLU values from the treatment plates after 3 days of MRTX1133 addition. Cell proliferation percent inhibition values were calculated by dividing luminescence unit values from each treated well by the average of the luminescence unit values in the vehicle-treated wells and multiplied by 100. Data were transposed and passed into GraphPad Prism to obtain IC50 values using a 4-parameter fit of the dose response curve. |
| Animal Protocol |
Tumor Pharmacodynamic and Tumor Xenograft Studies All mouse studies were conducted in compliance with all applicable regulations and guidelines of the Institutional Animal Care and Use Committee (IACUC) from the National Institutes of Health (NIH). Mice were maintained under pathogen-free conditions, and food and water were provided ad libitum. 6–8-weekold, female, athymic nude-Foxn1nu mice (Envigo, San Diego) were injected subcutaneously with Panc 04.03 cells in 100 l of PBS and Matrigel matrix in the right hind flank with 5.0 x 106 cells (Corning #356237; Discovery Labware, MA) 50:50 cells : Matrigel. Mouse health was monitored daily, and caliper measurements began when tumors were palpable. Tumor volume measurements were determined utilizing the formula 0.5 x L x W2 in which L refers to length and W refers to width of each tumor. Tumor pharmacodynamic studies: When tumors reached an average tumor volume of ~400 mm3 , mice were randomized into treatment groups. Mice were treated by intraperitoneal injection with either vehicle consisting of 10% research grade Captisol (CyDex Pharmaceuticals, KS) in 50 mM citrate buffer pH 5.0 or MRTX1133 at 30mg/kg. Tumors and plasma were collected at 1 hour and 12 hours after a single dose to determine exposure levels. Tumor fragments were snap frozen in homogenization tubes (Omni, #19-628-3) with liquid nitrogen and homogenized (MPBio FastPrep-24 system) with Lysis/Binding Buffer AM11 (Active Motif, #52097) with protease and phosphatase inhibitors added fresh before use. Tumor lysates were then assayed for ERK1/2 phosphorylation. Xenograft studies: When tumors reached an average tumor volume of ~350 mm3 , mice were randomized into treatment groups. Mice were treated by intraperitoneal injection with either vehicle consisting of 10% research grade Captisol (CyDex Pharmaceuticals, KS) in 50 mM citrate buffer pH 5.0 or MRTX1133 in vehicle at 3, 10, or 30mg/kg BID. Animals were monitored daily, tumors were measured 3 times per week, and body weights were measured 2 times per week. Data are expressed as mean +/- SEM. Statistical analysis of differences in mean tumor volume between vehicle and MRTX1133-treated cohorts was run using a two-tailed Student’s t-test with equal variance in Excel (Microsoft; Redmond, WA). Animal/Disease Models: 6-8-week age, female/athymic nude-Foxn1nu mice (Panc 04.03 model) Doses: 3, 10, 30 mg/kg Route of Administration: Intraperitoneal injection/ip; twice a day for 28 days Experimental Results: MRTX1133 dose-dependent suppressed tumor growth with a TGI of 94% observed at 3 mg/kg BID (IP) and TGIs of -62% and -73% observed at 10 and 30 mg/kg BID (IP), respectively. |
| References |
[1]. Front Oncol . 2021 May 3:11:672612. [2]. J Med Chem . 2022 Feb 24;65(4):3123-3133. [3]. Nat Med . 2022 Oct;28(10):2171-2182. |
| Additional Infomation | KRAS G12D Inhibitor MRTX1133 is an orally bioavailable reversible inhibitor of the oncogenic KRAS substitution mutation G12D, with potential antineoplastic activity. Upon oral administration, KRAS G12D inhibitor MRTX1133 specifically targets and noncovalently binds to KRAS G12D. This prevents KRAS G12D-mediated signaling and activation of downstream survival pathways. This leads to an inhibition of the growth of tumor cells that overexpress KRAS G12D. KRAS, a member of the RAS family of oncogenes, serves an important role in cell signaling, division and differentiation. Mutations of KRAS may induce constitutive signal transduction leading to tumor cell proliferation, invasion, and metastasis. |
Solubility Data
| Solubility (In Vitro) | DMSO:50~100 mg/mL (83.3~166.5 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 10 mg/mL (16.65 mM) in 10% SBE-β-CD/50 mM citrate pH 5.0 (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 2: 3.5 mg/mL (5.83 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 35.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (4.16 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 4: 5%DMSO+40%PEG300+5%Tween80+50%ddH2O: 25mg/ml (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6649 mL | 8.3246 mL | 16.6492 mL | |
| 5 mM | 0.3330 mL | 1.6649 mL | 3.3298 mL | |
| 10 mM | 0.1665 mL | 0.8325 mL | 1.6649 mL |